22/01/2024 BlogEye ConditionsNewsStem Cell Therapies
Stem Cell Blog
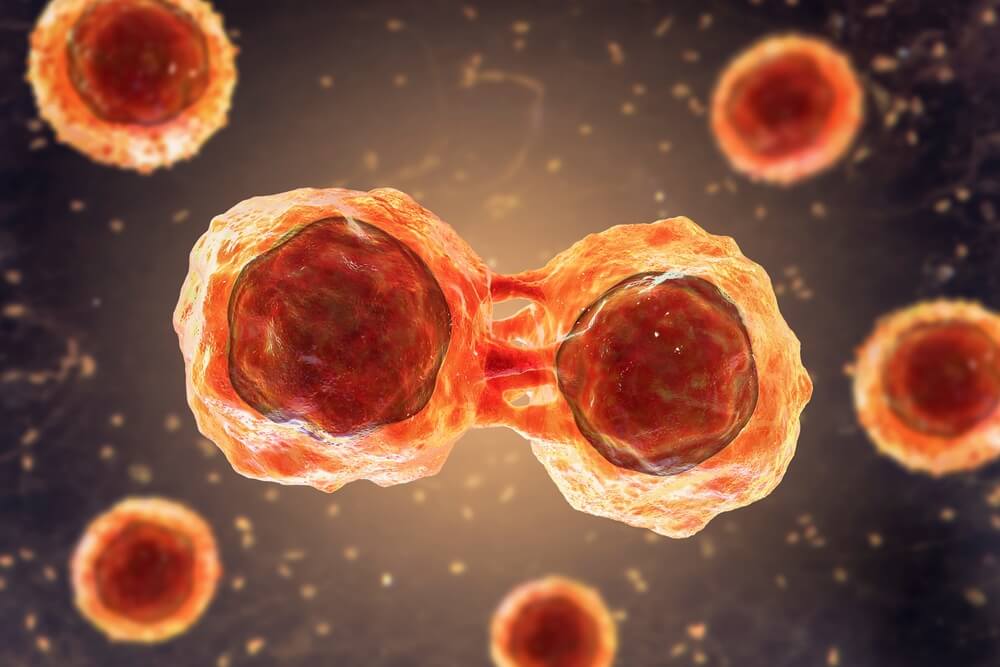
Употребата на матичните клетки од папочна врвца рапидно се зголемува. Пред 10 години крвта од папочна врвца можеше да лекува околу 40 состојби, но денес таа бројка е над 80. Со нетрпение очекуваме нови терапии за болести и нарушувања како што се дијабет, аутизам и мозочен удар, можете да бидете во тек со најновите случувања во регенеративната медицина на нашиот блог за матични клетки.
22/01/2024 BlogNewsPregnancy AdviceUses for Cord Blood
November 15th is World Cord Blood Day. This annual event was created to raise awareness of cord blood, what it is and some of the therapeutic benefits it can offer.
If you are curious to find out more, read on.
Stem cells are amazing. You may think this a biased opinion coming from us, but the following story is here to prove they are amazing. A young man who donated his stem cells so that a woman overseas could receive a lifesaving treatment gets to meet her.
20/01/2024 News