A phase 1 trial of a novel cell therapy for lymphoma that is highly resistant to standard treatment (highly refractory lymphoma) has published very positive results in the Nature Medicine journal. The therapy, which uses natural killer (NK) cells derived from cord blood, resulted in complete remission in two thirds of the treated patients.
What are natural killer cells?
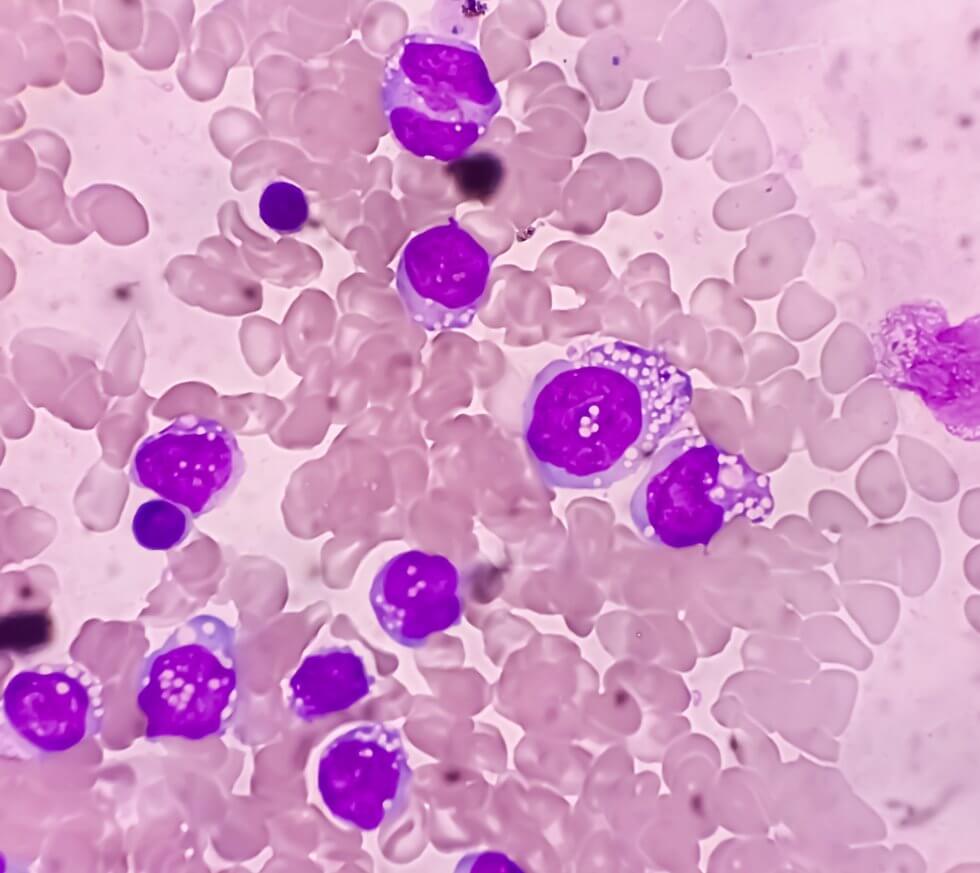
NK cells are a specific type of white blood cell, a part of the immune system which targets and destroys infected and cancerous cells. These cells patrol the body, determining which cells should be destroyed based on whether they receive the correct signals from them or not.[1]
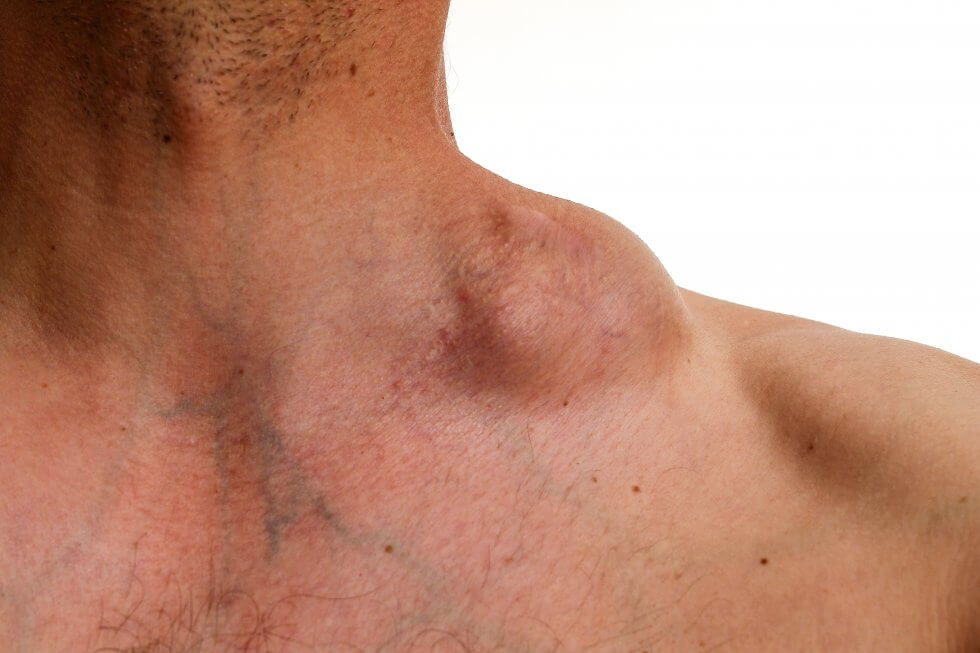
Their ability to kill cancerous cells makes NK cells ideal for the development of cell therapies to treat cancer. These typically hinge on modifying the NK cells, granting them the ability to recognise and destroy cancerous cells they would otherwise ignore.[2] Such is the case for this novel cord blood cell therapy for lymphoma, a type of blood cancer which affects lymphocytes, white blood cells which are part of the immune system. Normally, lymphocytes help the body fight off infection. With lymphoma, however, they do not work correctly and can multiply out of control, accumulating quickly in lymph nodes and other organs in the lymphatic system, such as the spleen and liver.[3][4]
Thanks to advances in medicine, lymphoma is generally considered a very treatable type of cancer, with 5-year survival rates averaging between 74% and 89% depending on the type of lymphoma.[5][6][7] However, sometimes the cancer still resists treatment or reoccurs afterwards, leaving patients with refractory or relapsed lymphoma with few options and a poor prognosis.[8] This can happen in between 10%-40% of cases, depending on the type of lymphoma, the patient age at diagnosis, and how advanced the disease was at diagnosis.[9][10] This is the need this cord blood cell therapy for lymphoma is attempting to fill.
What does the new therapy entail?
Researchers first isolated NK cells from cord blood and then activated them using a combination of cytokines.[8] Cytokines are proteins which the immune system uses to send signals;[11] using them to activate NK cells stimulates their cancer-fighting abilities.[12]
Then, the antibody AFM13 was added to the NK cells, enabling them to target the CD30 protein. This protein is found on the surface of cancerous cells in specific types of lymphoma, primarily in Hodgkin lymphoma but also other varieties.[8]
Following chemotherapy, doctors infused a dose of the treated NK cells into patients. This was then followed by three doses of the AFM13 antibody, administered once a week.
Why is this therapy so important?
The 42 patients who took part in the trial all had highly refractory lymphoma, having received a median of seven prior treatment courses. In spite of this, the therapy achieved an overall response rate of 92.9%, with 66.7% of patients experiencing complete remission. In patients with Hodgkin lymphoma specifically, the rates were even higher, with a complete remission rate of 73% and an overall response rate of 97.3%.
Eleven patients remained in complete remission for at least 14 months, with this lasting, for some, up to 40 months after receiving the therapy. Five patients remained in complete remission without any further treatment, and six went on to receive a stem cell transplant.
At a median follow-up of 20 months, the therapy led to complete disease remission in 26.2% patients, or about one in four patients. The two-year overall survival rate was 76.2%.[8] These are encouraging and very positive results, particularly considering how resistant their cancer had been to previous treatment. Moreover, the therapy proved to be safe and well-tolerated, with no adverse side effects beyond those caused by the chemotherapy.
This trial was a small-scale one, primarily aimed at confirming the safety of the treatment and determining an optimal dose. Larger trials are therefore needed before this therapy can become available to patients.
Still, trials like this, as well as many others currently ongoing, highlight the strong curative potential of cord blood cells. To discover more about cord blood cells, and find out how you could preserve them for your baby and family’s potential future use, fill in the form below to request our free guide.
References
[3] Blood Cancer UK. Lymphoma. https://bloodcancer.org.uk/understanding-blood-cancer/lymphoma/