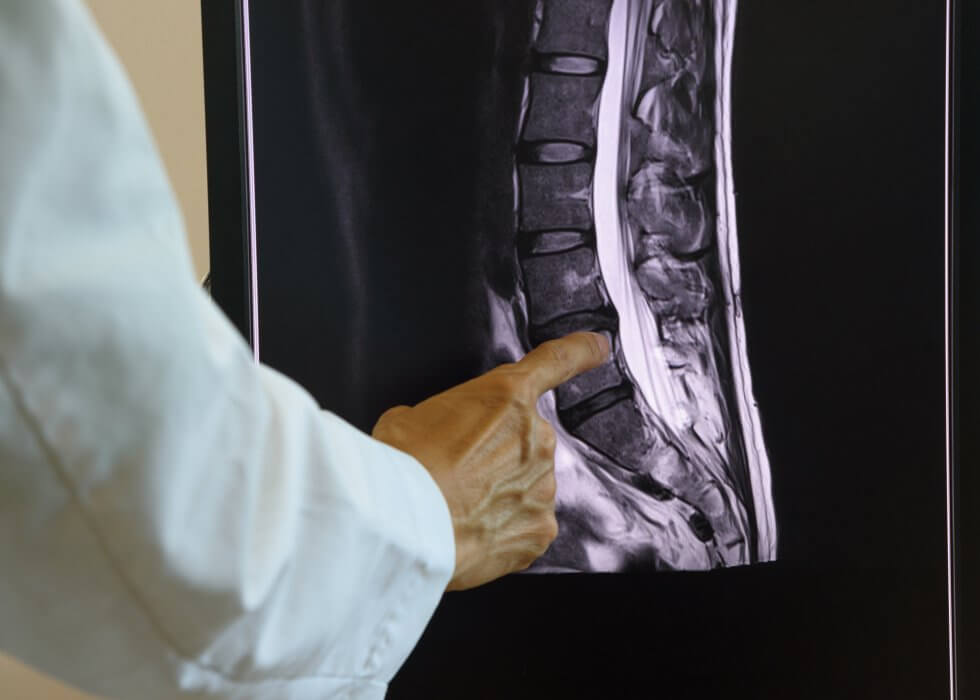
A recent study has found that a stem cell therapy could reduce epileptic symptoms after stroke and help the brain recover. The study was performed at the Gladstone Institute of Neurological Disease in California, USA, using a rat model of stroke.
Stroke basics
A stroke is a medical condition which causes the death of cells in the brain. This, in turn, stops the brain from working properly. There are two main types of stroke: haemorrhagic, caused by bleeding in the brain, and ischemic, caused by the blockage of a blood vessel. Ischemic stroke is by far the most common, accounting for just under 90% of strokes.[1]
Stroke is the second leading cause of death and one of the major causes of disability worldwide.[2] In the UK, it is the single biggest cause of severe disability, causing a range greater than any other condition, including movement issues, visual problems, and speech difficulties.[3]
The goal of the study
The stem cell therapy tested in the study is based on mesenchymal stem cells derived from donor bone marrow. It has already been successful in clinical trials for improving chronic paralysis after traumatic brain injury[4][5]; this led to its approval as a treatment for this issue in Japan[6].
Now, researchers are hoping it could also help reverse brain damage caused by stroke. In particular, they are focusing on brain hyperexcitability, which is a flawed response in the brain that develops in some people after they have had a stroke.
What is brain hyperexcitability?
Put simply, brain hyperexcitability is an increased chance for neurons to activate in response to a specific stimulus. In the case of stroke, this flawed response develops in the brain as it tries to make up for lost functions.
These overly active neurons send out signals that are too frequent or too strong to other regions of the brain. This, in turn, causes serious issues. Brain hyperexcitability can make it difficult to control muscles (spasticity) and can lead to seizures.[7] This means stroke is a leading cause of acquired epilepsy,[8] particularly in people over the age of 35.[9]
Unfortunately, brain hyperexcitability remains not well understood, and there is no treatment available to prevent it[7]. Post-stroke seizures and epilepsy are simply managed, as and when needed, with anti-epileptic drugs.[10]
Study process and findings
Researchers tested the stem cell therapy in a rat model of stroke. A month after rats had a stroke, modified human stem cells were injected into their brains, near the damaged area.
The team then measured electrical activity in the brain to determine the effectiveness of the therapy. Furthermore, they also analysed the structure of the brain and blood cells in detail, so they could study the changes wrought by the stroke and by the therapy.
They found that the stroke had caused hyperexcitability in the rats’ brains. The stem cell therapy, however, had reversed this, returning the brain to normal function. Furthermore, a number of proteins and cells that are important for brain function had also increased.
Additionally, by comparing rats which had received the therapy to control rats, researchers identified molecules in the blood that had changed after the stroke. They further saw that these same molecules were restored to normal by the therapy. Additional analysis found that a week after the transplant, few stem cells remained in the rats’ brain; however, the effects were long-lasting.
Hope for future treatment
This therapy is in the very early stages of research; it remains to be determined whether the reversal of brain hyperexcitability would lead to a reduction of symptoms in actual patients.
Still, studies like these highlight the tremendous regenerative potential of stem cells, and the hope they can offer for the treatment of illnesses and injuries that today are considered incurable.
Today, stroke occurs more than 100,000 times per year in the UK – about once every five minutes. Advances in immediate treatment have meant that the number of patients dying from stroke continues to decrease. Unfortunately, however, rehabilitation hasn’t kept pace, and the number of people living with severe disabilities after stroke continues to increase.[3]
Stem cell therapies for post-stroke disability could prove to be truly lifechanging.
Some of the most potent stem cells that could be used in regenerative therapy come your baby’s umbilical cord and placenta. Both of these are normally thrown away at birth – but they could instead be stored for your baby’s future use. If you want to find out more about this rich source of stem cells, and learn how you could preserve it, fill out the form below to download our free parent’s guide.
References
[1] National Heart, Lung and Blood Institute (2023). Stroke – What Is a Stroke? https://www.nhlbi.nih.gov/health/stroke
[2] Katan, M. and Luft, A. (2018). Global Burden of Stroke. Seminars in Neurology, [online] 38(02), pp.208–211. doi:https://doi.org/10.1055/s-0038-1649503
[3] Brain Research UK (2021). Stroke – Neurological condition. https://www.brainresearchuk.org.uk/neurological-conditions/stroke
[4] Kawabori, M. et al. (2021). Cell Therapy for Chronic TBI. Neurology, 96(8), pp.e1202–e1214. doi:https://doi.org/10.1212/wnl.0000000000011450
[5] Okonkwo, D.O. et al. (2024). Mesenchymal Stromal Cell Implants for Chronic Motor Deficits After Traumatic Brain Injury: Post Hoc Analysis of a Randomized Trial. Neurology, [online] 103(7), p.e209797. doi:https://doi.org/10.1212/WNL.0000000000209797
[6] Neuro Central. (2024). SanBio Obtains Marketing Approval for ‘AKUUGO® Suspension for Intracranial Implantation’ (INN: Vandefitemcel) as a Therapeutic Agent for Improving Chronic Motor Paralysis From Traumatic Brain Injury (TBI). https://www.neuro-central.com/sanbio-obtains-marketing-approval-for-akuugo-suspension-for-intracranial-implantation-inn-vandefitemcel-as-a-therapeutic-agent-for-improving-chronic-motor-paralysis-from-tra/
[7] Klein, B. et al. (2024). Modified human mesenchymal stromal/stem cells restore cortical excitability after focal ischemic stroke in rats. Molecular Therapy. doi:https://doi.org/10.1016/j.ymthe.2024.12.006
[8] Adhikari, Y., Ma, C.-G., Chai, Z. and Jin, X. (2023). Preventing development of post-stroke hyperexcitability by optogenetic or pharmacological stimulation of cortical excitatory activity. Neurobiology of Disease, 184, p.106233. doi:https://doi.org/10.1016/j.nbd.2023.106233
[9] Mayo Clinic (2021). Epilepsy – Symptoms and Causes. https://www.mayoclinic.org/diseases-conditions/epilepsy/symptoms-causes/syc-20350093
[10] Holtkamp, M., Beghi, E., Benninger, F., Kälviäinen, R., Rocamora, R. and Christensen, H. (2017). European Stroke Organisation guidelines for the management of post-stroke seizures and epilepsy. European Stroke Journal, 2(2), pp.103–115. doi:https://doi.org/10.1177/2396987317705536