BlogNeurological DisordersNewsStem Cell NewsStem Cell TherapiesUses for Cord BloodUses for Cord Tissue
Fresh Insights on Cell Therapy for Cerebral Palsy
24/08/2025

A new study on cell therapy for cerebral palsy has, for the first time, directly compared the efficacy of two different treatments. Although more research is needed, the study’s results offer valuable insights for future clinical trials tackling this challenging disorder.
The challenges of cell therapy for cerebral palsy
Over the past two decades, cell therapy has emerged as a promising treatment for cerebral palsy. In particular, mononuclear cells from cord blood (UCB-MNCs) and mesenchymal stem cells from cord tissue (UCT-MSCs) have proven to be a safe and effective therapy. Many clinical trials have obtained results showing improvements in patients’ motor function through these cells’ anti-inflammatory, neuroprotective and regenerative properties.
However, efforts to pinpoint the best treatment method have been stymied by significant variations in trial parameters. Differences from study to study can include the type and severity of cerebral palsy, the age of the patients, the cell type, dose and delivery method, whether the study is open-label or blinded, and even the methods and time periods used to monitor improvement. Consequently, no two studies are alike, and results cannot be directly compared.[1][2]
Study methods and goal
The new study, conducted in Iran, is a pooled analysis of the results of two studies, one on UCB-MNTs and one on UCT-MSCs. Both studies were conducted in the same research centre; moreover, to aid in the comparison, the research methodology and other variables were kept the same as much as possible.[3][4][5]
The study patients were aged 4-14 and had spastic cerebral palsy with white matter lesions. After eligibility screening, 108 patients were randomly assigned to the two treatment arms or a control group. Treatment was done via intrathecal (into the spinal fluid) injection, with the control group receiving a sham procedure instead. Researchers then assessed patients’ motor function, quality of life, disability and spasticity after 1, 3, 6 and 12 months.
Both individual studies were double-blinded. Furthermore, all statistical analysis, both for individual studies and for the final pooled comparison, was performed by a blinded statistician.
The individual studies aimed to confirm that UCB-MNCs and UCT-MSCs are a safe and effective treatment for cerebral palsy. Following that, the pooled study analysis compared the effects of the UCB-MNC treatment to those of the UCT-MSC treatment across the study period.
Study results
Both the UCB-MNC treatment and the UCT-MSC treatment reported positive results over time when compared to the control group. Patients in both groups showed improvement in gross motor function and quality of life, as well as reduction in disability and spasticity.[4][5]
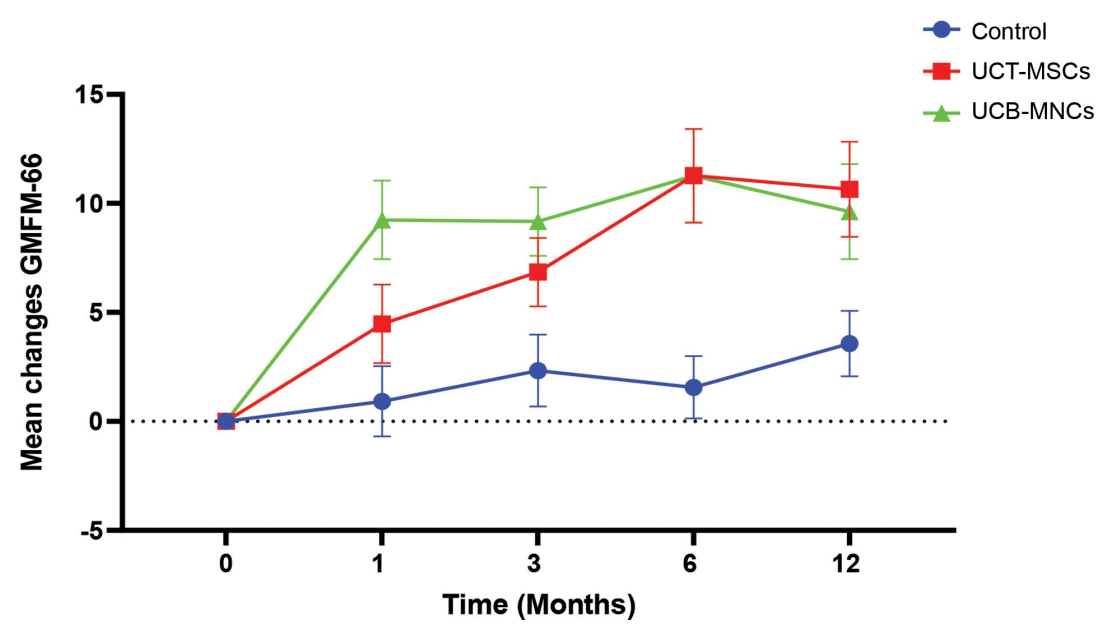
Figure 1. A comparison graph of the two different treatment groups and the control group.[3]