Stem Cell Blog
Употребата на матичните клетки од папочна врвца рапидно се зголемува. Пред 10 години крвта од папочна врвца можеше да лекува околу 40 состојби, но денес таа бројка е над 80. Со нетрпение очекуваме нови терапии за болести и нарушувања како што се дијабет, аутизам и мозочен удар, можете да бидете во тек со најновите случувања во регенеративната медицина на нашиот блог за матични клетки.
Researchers are investigating a novel placenta-derived stem cell therapy for multiple sclerosis, a currently incurable degenerative neurological condition. A recent phase 1 clinical trial, with findings published in Scientific Reports, assessed the safety and feasibility of this approach for patients with secondary progressive multiple sclerosis.
What is multiple sclerosis?
Multiple sclerosis is a lifelong condition that affects the brain and central nervous system. It is an autoimmune condition, caused by the immune system mistakenly attacking the myelin sheath which covers and protects the nerves. As a result of the myelin damage, the nerves become less efficient at sending messages to the body. The nerve damage continues to worsen over time, eventually leading to permanent disability.[1][2] Globally, multiple sclerosis affects around 2.3 million people.[3]
There are three main types of multiple sclerosis. In the relapsing-remitting type (RRMS), patients have distinct attacks (relapses) of symptoms, which then fade away partially or completely (remission). In the primary progressive type (PPMS), conversely, there is a gradual worsening of symptoms. Lastly, the secondary progressive type (SPMS) develops after RRMS for many people, and involves a gradual worsening of symptoms with or without active relapses.[4][5]
There is no cure for multiple sclerosis. Currently available treatment focuses on managing symptoms as well as reducing the seriousness and progression of the disease (disease-modifying therapies).[5][1]
Investigating stem cell therapy for multiple sclerosis
Current research is increasingly focusing on trying to find new treatments that would promote myelin regeneration (remyelination), reduce inflammation, and protect the nerves. Mesenchymal stem cells (MSCs) have the potential to address these goals, thanks to their anti-inflammatory, immunomodulatory, regenerative and neuroprotective properties. MSCs derived from the placenta (PL-MSCs), in particular, may have more potent immunosuppressive and immunomodulatory effects compared to other types of MSCs. What’s more, they can be obtained easily and non-invasively.[3]
Researchers posited that PL-MSCs may therefore have a positive impact on patients with SPMS, more specifically on those who no longer respond to conventional treatment (treatment-refractory).
The phase 1 clinical trial involved five patients with treatment-refractory SPMS, and was aimed primarily at determining the safety and tolerability of the PL-MSC treatment over a six-month period. Researchers also looked at exploratory secondary outcomes, including clinical disability, cognitive and psychological assessments, brain imaging (DTI and fMRI), and immunological markers.
What did the study find?
The treatment proved to be safe, with no serious complication occurring. Two patients had a mild headache, but this was resolved with a common painkiller.
Importantly, the results showed sustained improvements in clinical outcomes, with significant reductions in Expanded Disability Status Scale (EDSS) scores, a common measure of MS disability, in the first month after the treatment. By the third month, two participants had a continued reduction in their EDSS scores, while the other three remained stable.
After the treatment, participants also improved in cognitive and psychological tests. Functional MRI analysis suggested significant enhancements in brain connectivity and cognitive function. Blood tests also showed a decrease in the number of B cells, which are immune system cells involved in multiple sclerosis. Inflammatory proteins were found to have decreased, whereas anti-inflammatory protein levels increased.
The potential of placenta stem cells
It is important to keep in mind that this was a small phase 1 trial with a very limited number of participants, no control group and a relatively short follow-up period. The results are quite encouraging, but more research and larger-scale trials are needed to confirm the effectiveness of the treatment.
Nevertheless, studies like these highlight the strong potential of placenta stem cells, as well as other stem cells derived from birth-related tissues, to treat conditions and diseases that are currently considered incurable. Having ready access to a source of these powerful cells could make the difference in the future in terms of treatments that are still being discovered today. To learn more about how to bank your baby’s placenta and umbilical cord for their own future use, fill in the form below to receive your free guide.
References
[1] MS Trust (2022). What is MS? https://mstrust.org.uk/information-support/about-ms/what-is-ms
[4] MS Society (2019). Types of MS. https://www.mssociety.org.uk/about-ms/types-of-ms
[5] NHS (2022). Multiple Sclerosis. https://www.nhs.uk/conditions/multiple-sclerosis/
As the world population ages, osteoarthritis (OA), a degenerative, “wear-and-tear” form of arthritis which most often occurs during middle age and onwards,[1] becomes more common and therefore more burdensome. Knee osteoarthritis, in particular, is a major cause of work loss and disability.[2] Because of this, researchers have been seeking more effective treatments for the condition through studies and clinical trials. One such recent trial focused on injections of mesenchymal stem cells (MSCs) derived from the placenta.
What is knee osteoarthritis?
In a healthy knee, smooth articular cartilage cushions the bone ends, allowing easy movement, aided by shock-absorbing wedges of cartilage called the menisci.[3] Knee osteoarthritis involves the progressive breakdown of this cartilage, reducing the protective space and potentially leading to bone-on-bone contact. Consequently, the affected joint becomes painful and stiff. Bony growths may also develop, deforming the bones and joint and potentially further limiting range of motion.[4] Quality of life for sufferers is severely reduced as a result,[5] particularly as knee osteoarthritis often involves both knees.[6]
The knee is one of the most common joints to be affected by osteoarthritis,[7] which itself is the most common type of arthritis in the UK[4] and worldwide.[8] Osteoarthritis is a long-term, chronic condition, affecting hundreds of millions of people worldwide[2]. It is currently considered incurable,[4] mainly due to the inability of available treatments to reverse damage to the joint.[9]
Knee osteoarthritis treatment options
Current treatment strategies for knee osteoarthritis focus mainly on managing symptoms and improving function.[10] These commonly include lifestyle modifications like regular low-impact exercise and weight management, as well as the use of supportive devices such as braces and canes, to reduce the strain on the knee. Pain relief medication is also common, as is physical therapy.
In cases where conservative treatment does not work sufficiently, doctors then look at surgical interventions. These could include injections such as corticosteroids, for pain relief, or hyaluronic acid, to provide some cushioning for the joint.[9] In severe cases, doctors may consider joint reshaping or joint replacement surgery.[9][10]
Researchers are constantly exploring new treatments for osteoarthritis that might address the underlying joint changes more directly. Of particular interest is regenerative medicine, including the use of MSCs. These powerful stem cells can differentiate into specialised cell types, including bone (osteoblasts) and cartilage (chondrocytes). Moreover, they have self-renewal capabilities and can provide an immune-suppressive, anti-inflammatory effect.[11]
There are several sources of MSCs, with those derived from bone marrow and fat having been investigated more so than those derived from perinatal (birth-related) tissues, such as the placenta and umbilical cord. This gap in the research is what this recent study aimed to address.[11]
What did the study find?
The primary goal of the study was to assess the safety and effectiveness of repeated placenta stem cell injections for knee osteoarthritis.[11]
The study enrolled a total of 26 patients who had been diagnosed with moderate to severe knee osteoarthritis. The participants were divided into two groups – a treatment group and a control. The treatment group received a series of three injections of placenta MSCs and hyaluronic acid directly into the knee joint, spaced four weeks apart. The control group, on the other hand, received only hyaluronic acid, at the same interval.
Researchers then assessed patients at the 6- and 12-month mark following the first injection. Progress was tracked using self-reported questionnaires measuring pain (VAS) and functionality (WOMAC), as well as through an MRI. Blood samples were also analysed for signs of inflammation.
Results showed that the injections of placenta MSCs were safe. Some patients experienced temporary joint pain or swelling after the injections. However, these issues resolved within a week and there were no serious side effects. In terms of effectiveness, the group receiving the stem cell treatment reported improvements in pain and joint function compared to the control group at both the 6- and 12-month mark. MRI analysis found no differences in cartilage thickness between the groups. Blood sample analysis, on the other hand, found reduced signs of inflammation in the treatment group, indicating the treatment had an anti-inflammatory effect.
Future treatment potential
It is important to realize that more extensive research is needed to confirm the effectiveness of placenta stem cell injections for knee osteoarthritis. Although very promising, the results come from a small-scale, phase 1 trial. Moreover, the trial was open-label, meaning patients were aware of whether they were receiving the MSC treatment. As a result, reporting of improvements could have been biased in favour of it.
The placenta stem cells used in this study are not the only promising treatment being investigated for osteoarthritis. In a separate study, for instance, cord blood stem cells were shown to help regenerate stronger knee cartilage. In the future, these powerful perinatal stem cells could provide an effective treatment not just for osteoarthritis, but also for many other illnesses and conditions currently considered incurable. To learn more about how you could preserve these stem cells for your baby, fill in the form below to receive your free guide.
References
[4] NHS (2023). Osteoarthritis. https://www.nhs.uk/conditions/osteoarthritis/
[6] NHS (2019). Symptoms – Osteoarthritis. https://www.nhs.uk/conditions/osteoarthritis/symptoms/
[10] NHS (2023). Treatment – Osteoarthritis. https://www.nhs.uk/conditions/osteoarthritis/treatment/
A phase 1 trial of a novel cell therapy for lymphoma that is highly resistant to standard treatment (highly refractory lymphoma) has published very positive results in the Nature Medicine journal. The therapy, which uses natural killer (NK) cells derived from cord blood, resulted in complete remission in two thirds of the treated patients.
What are natural killer cells?
NK cells are a specific type of white blood cell, a part of the immune system which targets and destroys infected and cancerous cells. These cells patrol the body, determining which cells should be destroyed based on whether they receive the correct signals from them or not.[1]
Their ability to kill cancerous cells makes NK cells ideal for the development of cell therapies to treat cancer. These typically hinge on modifying the NK cells, granting them the ability to recognise and destroy cancerous cells they would otherwise ignore.[2] Such is the case for this novel cord blood cell therapy for lymphoma, a type of blood cancer which affects lymphocytes, white blood cells which are part of the immune system. Normally, lymphocytes help the body fight off infection. With lymphoma, however, they do not work correctly and can multiply out of control, accumulating quickly in lymph nodes and other organs in the lymphatic system, such as the spleen and liver.[3][4]
Thanks to advances in medicine, lymphoma is generally considered a very treatable type of cancer, with 5-year survival rates averaging between 74% and 89% depending on the type of lymphoma.[5][6][7] However, sometimes the cancer still resists treatment or reoccurs afterwards, leaving patients with refractory or relapsed lymphoma with few options and a poor prognosis.[8] This can happen in between 10%-40% of cases, depending on the type of lymphoma, the patient age at diagnosis, and how advanced the disease was at diagnosis.[9][10] This is the need this cord blood cell therapy for lymphoma is attempting to fill.
What does the new therapy entail?
Researchers first isolated NK cells from cord blood and then activated them using a combination of cytokines.[8] Cytokines are proteins which the immune system uses to send signals;[11] using them to activate NK cells stimulates their cancer-fighting abilities.[12]
Then, the antibody AFM13 was added to the NK cells, enabling them to target the CD30 protein. This protein is found on the surface of cancerous cells in specific types of lymphoma, primarily in Hodgkin lymphoma but also other varieties.[8]
Following chemotherapy, doctors infused a dose of the treated NK cells into patients. This was then followed by three doses of the AFM13 antibody, administered once a week.
Why is this therapy so important?
The 42 patients who took part in the trial all had highly refractory lymphoma, having received a median of seven prior treatment courses. In spite of this, the therapy achieved an overall response rate of 92.9%, with 66.7% of patients experiencing complete remission. In patients with Hodgkin lymphoma specifically, the rates were even higher, with a complete remission rate of 73% and an overall response rate of 97.3%.
Eleven patients remained in complete remission for at least 14 months, with this lasting, for some, up to 40 months after receiving the therapy. Five patients remained in complete remission without any further treatment, and six went on to receive a stem cell transplant.
At a median follow-up of 20 months, the therapy led to complete disease remission in 26.2% patients, or about one in four patients. The two-year overall survival rate was 76.2%.[8] These are encouraging and very positive results, particularly considering how resistant their cancer had been to previous treatment. Moreover, the therapy proved to be safe and well-tolerated, with no adverse side effects beyond those caused by the chemotherapy.
This trial was a small-scale one, primarily aimed at confirming the safety of the treatment and determining an optimal dose. Larger trials are therefore needed before this therapy can become available to patients.
Still, trials like this, as well as many others currently ongoing, highlight the strong curative potential of cord blood cells. To discover more about cord blood cells, and find out how you could preserve them for your baby and family’s potential future use, fill in the form below to request our free guide.
References
[3] Blood Cancer UK. Lymphoma. https://bloodcancer.org.uk/understanding-blood-cancer/lymphoma/
Getting older is not just about grey hair and wrinkles. The body can deteriorate, losing muscle tone and strength, making daily life activities more difficult and tiring. What’s more, the immune system also weakens, making older adults more likely to get sick and less able to fight off infection. Because of this, preventing or reversing the effects of ageing is the key to lifelong health. Although lifestyle changes and exercise can help offset some of these effects[1], researchers are increasingly looking at umbilical cord stem cells for their protective and restorative potential.
Protecting the immune system: the thymus and spleen
The thymus and spleen are both part of the lymphatic system, which itself is part of the body’s immune system. The thymus is where a type of white blood cells, called T cells, fully mature; these cells control the body’s immune response and attack infected and cancerous cells. The spleen, on the other hand, mainly acts like a filter, removing old or damaged blood cells from the body. It also helps produce white blood cells and destroy bacteria and viruses.[2] Changes in the thymus and spleen during a person’s lifetime mean the immune system gradually becomes less effective, making the person more vulnerable to many illnesses, including infections and cancer.[3][4]
A recent study, performed on a mouse model of ageing, showed that mesenchymal stem cells derived from umbilical cord tissue (UCT-MSCs) could prevent ageing-related changes in the thymus and spleen. The study compared three groups of mice: a control group, an induced-ageing group which received no treatment, and an induced-ageing group which also received treatment with human UCT-MSCs. Upon analysis, the thymus and spleen in the UCT-MSC treatment group were found to more closely resemble those in the control group in size, structure, and potential functionality. Those in the ageing group with no treatment, on the other hand, were significantly atrophied. Moreover, mice in the UCT-MSC treatment group also showed lower levels of inflammation and oxidative stress, lower expression of ageing-related genes, and an improved population of beneficial bacteria in the digestive system.[5]
Strengthening muscles and easing frailty
Losing some muscle mass and strength is a natural part of ageing. In some people, however, the loss becomes so significant the muscles atrophy, a condition called age-related sarcopenia. This condition can make even the simplest task, such as getting out of chairs or climbing stairs, quite difficult. Sarcopenia can also be a complicating factor leading to frailty, which increases the risk of falls, broken bones, disability and death.[6][7] Here, too, UCT-MSCs could prove beneficial as a treatment.
A pre-clinical study conducted on mice, specifically on sarcopenia, found that treatment with human UCT-MSCs could counteract muscle ageing. Mice in the treatment group showed improvements in grip strength and performed better in a fatigue test compared to the group which received no treatment. Furthermore, muscle analysis and comparison between groups showed restored muscle functionality in the treatment group.[8]
A different, phase 1/2 clinical trial on elderly patients with diagnosed frailty reported similar results. The study was randomised and double-blinded, and aimed to test both the safety and the effectiveness of UCT-MSC treatment. After a first, baseline evaluation, patients were randomly assigned to either the treatment or the control group. Following two infusions of either UCT-MSCs or placebo at days 1 and 30, they were then evaluated with several follow-up visits over the course of six months. Compared to the control group, patients treated with UCT-MSCs showed improvements in general quality of life, grip strength and walking ability.[9]
Protecting the future, today
Although more research is definitely needed, studies such as these highlight the strong regenerative potential of stem cells from the umbilical cord.
Babies being born today could be part of the first generation to live beyond 100. Having access to their own umbilical cord stem cells could help them gain access to anti-ageing treatments and remain in good health.
To learn more about how to preserve this powerful resource for your baby, as well as more potential uses for umbilical cord and placenta stem cells, complete the form below to request your free guide.
References
Osteoarthritis, a chronic degenerative joint disease, affects millions of people worldwide and is a leading cause of disability.[1][2] Currently available treatments can offer relief from pain and other symptoms. However, they fail to address the root of the disease, as they cannot effectively restore the damaged joint.[3] A novel stem cell therapy based on cord blood mesenchymal stem cells (UCB-MSCs) is now emerging as a promising regenerative approach. The therapy, called Cartistem, has been shown to regenerate strong, elastic cartilage in the joint, rather than the fibrous, weaker cartilage created by an alternative treatment.[4]
What is osteoarthritis?
Osteoarthritis is the most prevalent form of arthritis, and primarily affects middle-aged and older adults. It involves the deterioration of the cartilage that protects the ends of bones within a joint. Because cartilage does not contain blood vessels, it has a limited ability to self-repair[5]; with continued deterioration, it disappears entirely, leading to damage and changes to the bones themselves. Symptoms include joint stiffness and pain, as well as reduced range of motion, and can increase over time. Eventually, they can become severe enough to make day-to-day activities more difficult.[1][6]
The risk of osteoarthritis increases with age, although there are other risk factors which can contribute. These include joint injuries, overuse of the joint in work or sports, obesity and genetics.[1] Osteoarthritis can affect any joint in the body, but most frequently appears in the hands, knees, hips and spine.[1] Knee osteoarthritis, in particular, often involves both knees. It can cause pain during walking, especially going uphill or downhill, as well as difficulty straightening the legs and a sensation of the knee “giving way”.[7]
Treatments for knee osteoarthritis
The current standard of care for knee osteoarthritis includes a range of treatments that aim to manage pain, reduce stiffness and improve range of motion. They include both surgical and non-surgical options.
Non-surgical treatment is typically tried at first. This includes pain medication as well as lifestyle changes, such as weight loss and exercise, and assistive devices, such as special footwear, braces, or walking aids.[8][9] If non-surgical treatments have not been effective or the patient’s daily life is seriously affected, doctors then look at surgical treatments. This could be an operation called an osteotomy, where a small section of bone on one side of the joint is removed to realign the joint and shift weight away from damaged parts of the cartilage.[10] Knee replacement, either partial or total, is also an option.[8][9]
Another possibility is a surgical treatment called microfracture or microdrilling. This treatment involves doctors making small holes into the surface of the bone where cartilage is absent. The holes cause blood and bone marrow to coat the surface of the bone, then forming into a clot. Thanks to the stem cells contained in blood and bone marrow, this clot eventually develops into a new layer of cartilage.[11] However, this new cartilage is not as strong as the original cartilage, and can break down more easily,[5][12] meaning any improvement is temporary.
A new therapeutic option
Cartistem is an off-the-shelf stem cell therapy developed in South Korea, where it has been approved for knee osteoarthritis treatment since 2012.[13] It consists of a combination of allogeneic UCB-MSCs and hyaluronic acid hydrogel. Applied to the area of damaged cartilage during surgery, the stem cells stimulate cartilage regeneration.[14]
Although Cartistem can be considered comparable to microdrilling in terms of the underlying process, a recent study has shown that this therapy can regenerate cartilage that is stronger and more resilient. The study, the results of which were recently presented at the 2025 annual meeting of the American Academy of Orthopaedic Surgeons, directly compared the results of Cartistem to those of microdrilling.[4]
According to researchers, Cartistem repaired a greater surface area than microdrilling, producing stronger cartilage which contained more collagen and had improved stiffness and elasticity. In contrast, microdrilling produced weaker, more fibrous cartilage, and in one out of five patients resulted in no cartilage regeneration at all.[14]
On the strength of the results from this and other studies, the FDA granted approval to proceed directly to a phase 3 clinical trial in the United States. A phase 3 trial is also underway in Japan.[4]
The regenerative power of cord blood stem cells
The strong potential of UCB-MSCs for the treatment of osteoarthritis is well-documented, with several recent studies highlighting their positive effects.[15][16][17]
Cartistem is a proprietary, off-the-shelf therapy, using allogeneic (donor) UCB-MSCs. However, as therapies are still being discovered and developed, it is entirely possible that a patient having access to their own cord blood stem cells could make the difference in terms of effectiveness. This could be the case not only for osteoarthritis, but for also for many other illnesses and diseases.
To discover more about cord blood stem cells, and how you could preserve them for your baby for potential future treatment, fill in the form below to request your free guide to cord blood banking.
References
[7] NHS (2019). Symptoms – Osteoarthritis. https://www.nhs.uk/conditions/osteoarthritis/symptoms/
[8] NHS (2023). Treatment – Osteoarthritis. https://www.nhs.uk/conditions/osteoarthritis/treatment/
[13] MEDIPOST. (2022). CARTISTEM®. https://en.medi-post.co.kr/cartistem/
A new study on cell therapy for cerebral palsy has, for the first time, directly compared the efficacy of two different treatments. Although more research is needed, the study’s results offer valuable insights for future clinical trials tackling this challenging disorder.
The challenges of cell therapy for cerebral palsy
Over the past two decades, cell therapy has emerged as a promising treatment for cerebral palsy. In particular, mononuclear cells from cord blood (UCB-MNCs) and mesenchymal stem cells from cord tissue (UCT-MSCs) have proven to be a safe and effective therapy. Many clinical trials have obtained results showing improvements in patients’ motor function through these cells’ anti-inflammatory, neuroprotective and regenerative properties.
However, efforts to pinpoint the best treatment method have been stymied by significant variations in trial parameters. Differences from study to study can include the type and severity of cerebral palsy, the age of the patients, the cell type, dose and delivery method, whether the study is open-label or blinded, and even the methods and time periods used to monitor improvement. Consequently, no two studies are alike, and results cannot be directly compared.[1][2]
Study methods and goal
The new study, conducted in Iran, is a pooled analysis of the results of two studies, one on UCB-MNTs and one on UCT-MSCs. Both studies were conducted in the same research centre; moreover, to aid in the comparison, the research methodology and other variables were kept the same as much as possible.[3][4][5]
The study patients were aged 4-14 and had spastic cerebral palsy with white matter lesions. After eligibility screening, 108 patients were randomly assigned to the two treatment arms or a control group. Treatment was done via intrathecal (into the spinal fluid) injection, with the control group receiving a sham procedure instead. Researchers then assessed patients’ motor function, quality of life, disability and spasticity after 1, 3, 6 and 12 months.
Both individual studies were double-blinded. Furthermore, all statistical analysis, both for individual studies and for the final pooled comparison, was performed by a blinded statistician.
The individual studies aimed to confirm that UCB-MNCs and UCT-MSCs are a safe and effective treatment for cerebral palsy. Following that, the pooled study analysis compared the effects of the UCB-MNC treatment to those of the UCT-MSC treatment across the study period.
Study results
Both the UCB-MNC treatment and the UCT-MSC treatment reported positive results over time when compared to the control group. Patients in both groups showed improvement in gross motor function and quality of life, as well as reduction in disability and spasticity.[4][5]
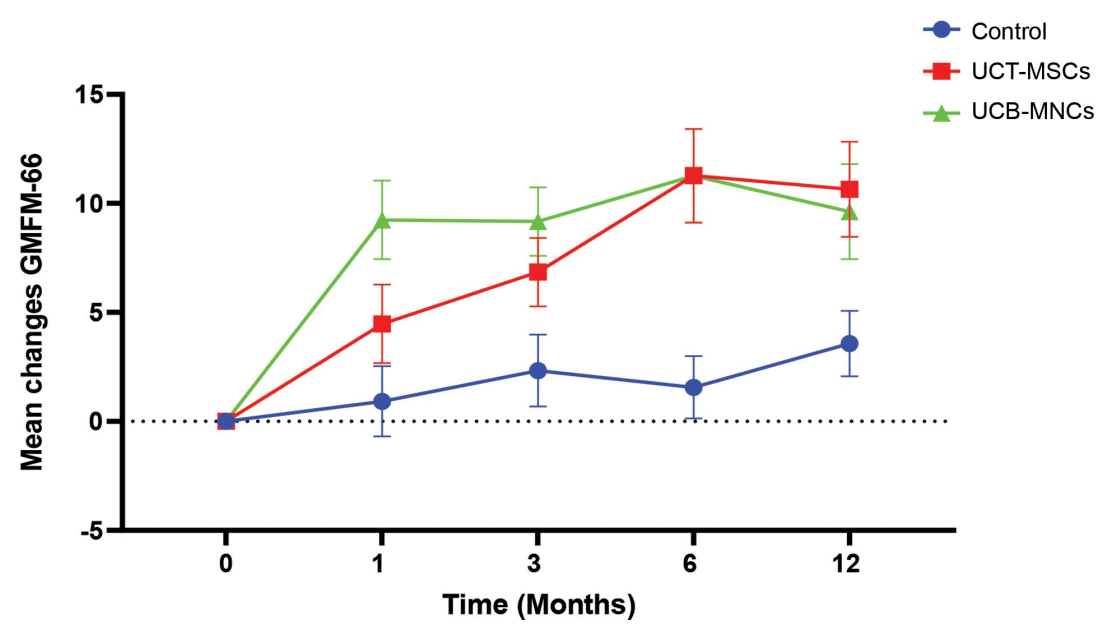
Figure 1. A comparison graph of the two different treatment groups and the control group.[3]
As the figure above shows, researchers found that the UCB-MNC treatment group showed stronger improvements in motor function early on. However, both the UCB-MNC and UCT-MSC groups achieved the same level of improvement at 6 months post treatment. At twelve months after treatment, there was some gradual deterioration of the improvements; however, researchers noted that UCT-MSCs did seem to result in more sustainable changes, with patients seeing less deterioration compared to the UCB-MNC group.[3]
Due to this deterioration, researchers posit that repeated doses at regular intervals may be the best route to continued improvement.[1] They also suggest that future trials should investigate treatments combining both UCB-MNCs and UCT-MSCs, as it may prove more effective than individual ones.[3]
The future of medicine
Researchers stress that this study is only a start, and further comparative trials and research are needed. Although both UCB-MNCs and UCT-MSCs offer positive results, there is still no certainty on which cell type and source will prove most effective treatment. This is true not only for cerebral palsy, but also for other illnesses and diseases for which an effective treatment is still being sought.
This uncertainty highlights the importance of comprehensive stem cell banking. By storing as many stem cell sources as possible, you could equip your baby and family with the broadest range of options for future regenerative therapies.
To find out how you could preserve both cord blood and tissue, along with amnion and placenta, for your baby’s potential future use, fill in the form below to request your free guide.
References
After an acid attack that “melted” his left eye, Paul Laskey thought he would lose his sight entirely. Now, a series of amnion grafts from a donated placenta has saved his eye, stabilising it and preventing further damage.[1]
A long road to recovery
Mr Laskey, from Newcastle, had acid squirted over his face after confronting a mugger, in February 2023. After he was rushed to hospital, doctors found that the chemical burn had, in effect, “melted” his left eye. The inner and outer layers of his cornea both suffered severe damage; the burn had caused limbal stem cell failure and severe neurotrophic keratopathy. In other words, the eye couldn’t repair or regenerate the cells within the cornea. Furthermore, the eye nerves didn’t work correctly.[2]
Doctors feared Mr Laskey would lose his eye entirely. First, they began by cleaning the eye, removing any remnants of the acid that might have still been there.[3] Then, over the following eight months, Mr Laskey underwent several surgeries, including two cornea transplants.
He also received three amnion grafts to stabilise his eye and help it heal, stopping his sight from deteriorating further. The first graft was a week after the attack, and the last more than a year later, in June 2024.[4]
Amnion grafts and eye injury
Amnion grafts are made from placenta – more specifically, from the amniotic membrane, the thin membrane on the inner side of the placenta which forms the amniotic sac. The amnion has anti-bacterial, anti-inflammatory and anti-scarring properties.[5]
Mothers who deliver via elective C-section at specific hospitals can choose to donate their placenta to NHS Blood & Transplant.[6] Scientists then use these to create specialised tissue grafts – between 50 and 100 from each donated placenta. These grafts are used to treat eye injuries, as well as burns and wounds.[3]
Ophthalmologist prof. Francisco Figueredo, who treated Mr Laskey, said that amnion grafts have been essential in helping the management of his severe eye burn.[3] Having saved his eye and preserved the vision he still has, doctors are now hoping to restore more of his eyesight through stem cell treatment.[1]
The future of placental therapy
Paul Laskey’s story unquestionably highlights the remarkable potential of the placenta and amnion. In addition to treatment for eye injuries, amnion grafts have also helped to save limbs from amputation and aided in facial reconstruction.
Although the regenerative properties of the amniotic membrane have been known for some time, research continues to explore the possible uses of the placenta and its components. Undoubtedly, only time will tell what other potentially life-saving treatments it could be used for.
Amnion patches, for instance, have been used to treat patients with inflammatory heart conditions[7]. Researchers have also used mesenchymal stem cells from the amniotic membrane to 3D print muscles, bone fragments, and even ears[8]. The placenta itself is also rich in mesenchymal stem cells, which are being studied to treat illnesses ranging from Crohn’s disease[9] to osteoarthritis[10].
To learn more about the potential uses of the placenta and other birth tissues, and how you could preserve them for your baby and family’s use in years to come, fill in the form below to receive your free guide.
References
A recent study has found that a stem cell therapy could reduce epileptic symptoms after stroke and help the brain recover. The study was performed at the Gladstone Institute of Neurological Disease in California, USA, using a rat model of stroke.
Stroke basics
A stroke is a medical condition which causes the death of cells in the brain. This, in turn, stops the brain from working properly. There are two main types of stroke: haemorrhagic, caused by bleeding in the brain, and ischemic, caused by the blockage of a blood vessel. Ischemic stroke is by far the most common, accounting for just under 90% of strokes.[1]
Stroke is the second leading cause of death and one of the major causes of disability worldwide.[2] In the UK, it is the single biggest cause of severe disability, causing a range greater than any other condition, including movement issues, visual problems, and speech difficulties.[3]
The goal of the study
The stem cell therapy tested in the study is based on mesenchymal stem cells derived from donor bone marrow. It has already been successful in clinical trials for improving chronic paralysis after traumatic brain injury[4][5]; this led to its approval as a treatment for this issue in Japan[6].
Now, researchers are hoping it could also help reverse brain damage caused by stroke. In particular, they are focusing on brain hyperexcitability, which is a flawed response in the brain that develops in some people after they have had a stroke.
What is brain hyperexcitability?
Put simply, brain hyperexcitability is an increased chance for neurons to activate in response to a specific stimulus. In the case of stroke, this flawed response develops in the brain as it tries to make up for lost functions.
These overly active neurons send out signals that are too frequent or too strong to other regions of the brain. This, in turn, causes serious issues. Brain hyperexcitability can make it difficult to control muscles (spasticity) and can lead to seizures.[7] This means stroke is a leading cause of acquired epilepsy,[8] particularly in people over the age of 35.[9]
Unfortunately, brain hyperexcitability remains not well understood, and there is no treatment available to prevent it[7]. Post-stroke seizures and epilepsy are simply managed, as and when needed, with anti-epileptic drugs.[10]
Study process and findings
Researchers tested the stem cell therapy in a rat model of stroke. A month after rats had a stroke, modified human stem cells were injected into their brains, near the damaged area.
The team then measured electrical activity in the brain to determine the effectiveness of the therapy. Furthermore, they also analysed the structure of the brain and blood cells in detail, so they could study the changes wrought by the stroke and by the therapy.
They found that the stroke had caused hyperexcitability in the rats’ brains. The stem cell therapy, however, had reversed this, returning the brain to normal function. Furthermore, a number of proteins and cells that are important for brain function had also increased.
Additionally, by comparing rats which had received the therapy to control rats, researchers identified molecules in the blood that had changed after the stroke. They further saw that these same molecules were restored to normal by the therapy. Additional analysis found that a week after the transplant, few stem cells remained in the rats’ brain; however, the effects were long-lasting.
Hope for future treatment
This therapy is in the very early stages of research; it remains to be determined whether the reversal of brain hyperexcitability would lead to a reduction of symptoms in actual patients.
Still, studies like these highlight the tremendous regenerative potential of stem cells, and the hope they can offer for the treatment of illnesses and injuries that today are considered incurable.
Today, stroke occurs more than 100,000 times per year in the UK – about once every five minutes. Advances in immediate treatment have meant that the number of patients dying from stroke continues to decrease. Unfortunately, however, rehabilitation hasn’t kept pace, and the number of people living with severe disabilities after stroke continues to increase.[3]
Stem cell therapies for post-stroke disability could prove to be truly lifechanging.
Some of the most potent stem cells that could be used in regenerative therapy come your baby’s umbilical cord and placenta. Both of these are normally thrown away at birth – but they could instead be stored for your baby’s future use. If you want to find out more about this rich source of stem cells, and learn how you could preserve it, fill out the form below to download our free parent’s guide.
References
Scientists have shown that muscle patches grown from stem cells can be used to strengthen and help repair a failing heart. In a breakthrough clinical trial, ten patches containing 400 million cells were implanted on the heart of a 46-year-old woman who was suffering from heart failure. The results, along with results from earlier studies which tested the same procedure in monkeys, have been published in Nature. [1]
What is heart failure?
Heart failure is when the heart is too weak or stiff and, as a result, cannot pump blood around the body as well as it should. It can have a variety of causes, from heart disease and heart attacks to high blood pressure and inflammation.[2][3]
Heart failure is a long-term condition which gradually gets worse over time. Currently, it is considered an incurable condition; it can only be treated to keep the symptoms under control with medication and, sometimes, surgery. In severe cases of heart failure, a heart transplant may be necessary. However, there is a shortage of hearts for transplantation, so patients may have to wait several years before one becomes available.[4]
Development of the therapy
The team of researchers, led by Prof. Zimmermann from University Medical Center Göttingen, Germany, coaxed stem cells to grow into heart muscle and connective tissue cells. They then mixed these cells with collagen gel to create patches which could be applied to the outside surface of the heart using a minimally invasive surgery.
After initial studies in vitro and in small animal models of heart failure confirmed the treatment had potential, the patches were first tested in monkeys. The team implanted the patches into six rhesus macaques with heart failure. Three of the monkeys received two patches, while the other three received five. These monkeys were also all treated with immunosuppressive drugs. A second group of seven monkeys remained untreated as a control.
The implanted cells remained smaller than the monkeys’ own heart muscle cells. However, the patches led to an improvement in heart function compared to the control monkeys, thickening the heart’s muscle and increasing its pumping power.[5]
First in-human trial
The success of the trial in monkeys led to the approval of a first-in-human phase 1/2 trial, called BioVAT-HF, which began in 2021 and is currently ongoing.[6][7][8]
The trial has so far recruited 19 patients. The first of these, a 46-year-old woman, had severe heart failure and was waiting for a heart transplant.[1] The researcher team implanted the muscle patches on her heart; she also received immunosuppressive drugs of the same type normally used for transplants. Three months later, the patient was lucky enough to be the recipient of a successful heart transplant.
Upon analysis of her old heart, scientists found that the implanted patches had survived and had formed blood vessels. In other words, they had integrated with the heart without any side effects.
The therapy is still in the early stages of research, and Zimmermann is very clear that it is not yet a replacement for a heart transplant. Rather, it is a supportive treatment for patients in advanced stages of heart failure, who are waiting for a transplant and are under palliative care.
Still, these are incredibly exciting results, which could prove to be a game-changer for the treatment of heart failure. The research team is continuing the clinical trial. In addition, they are testing new patch designs in monkeys in hopes of minimising the need for immunosuppressive drugs.
Stem cells: the future of medicine
This study is one more example of how stem cells are shaping research through their applications in regenerative medicine.
The stem cells used in this particular clinical trial are allogeneic (donor) induced pluripotent stem cells (iPSCs). When receiving a donor transplant, there is always a risk of rejection, hence the need for immunosuppressive drugs.
Your baby’s umbilical cord is a rich source of stem cells, among the most naïve and potent your baby will ever have. They could be used for similar therapies in the future, should your baby need them. What’s more, they could be used without risk of rejection: they are your baby’s own cells, their own perfect genetic match.
You only get one opportunity to collect and store these cells: in the 15 minutes after your baby is born. To find out more about how you can preserve this precious resource, fill in the form below to request your free guide to cord blood banking.
References
[2] NHS (2022). Heart failure. https://www.nhs.uk/conditions/heart-failure/
[4] NHS (2022). Treatment – Heart Failure. https://www.nhs.uk/conditions/heart-failure/treatment/
[8] Dzhk.de. (2025). DZHK-Studie BioVAT-HF-DZHK20. https://biovat.dzhk.de