Stem Cell Blog
Употребата на матичните клетки од папочна врвца рапидно се зголемува. Пред 10 години крвта од папочна врвца можеше да лекува околу 40 состојби, но денес таа бројка е над 80. Со нетрпение очекуваме нови терапии за болести и нарушувања како што се дијабет, аутизам и мозочен удар, можете да бидете во тек со најновите случувања во регенеративната медицина на нашиот блог за матични клетки.
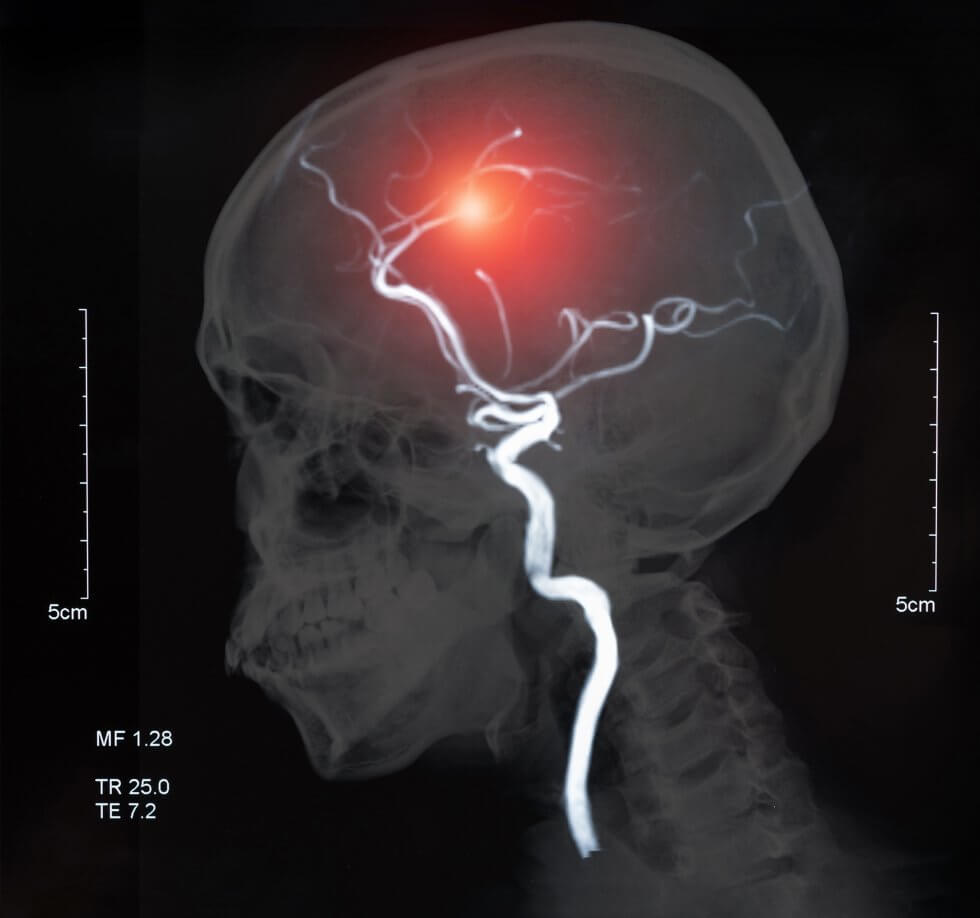
Scientists have shown that muscle patches grown from stem cells can be used to strengthen and help repair a failing heart. In a breakthrough clinical trial, ten patches containing 400 million cells were implanted on the heart of a 46-year-old woman who was suffering from heart failure. The results, along with results from earlier studies which tested the same procedure in monkeys, have been published in Nature. [1]
What is heart failure?
Heart failure is when the heart is too weak or stiff and, as a result, cannot pump blood around the body as well as it should. It can have a variety of causes, from heart disease and heart attacks to high blood pressure and inflammation.[2][3]
Heart failure is a long-term condition which gradually gets worse over time. Currently, it is considered an incurable condition; it can only be treated to keep the symptoms under control with medication and, sometimes, surgery. In severe cases of heart failure, a heart transplant may be necessary. However, there is a shortage of hearts for transplantation, so patients may have to wait several years before one becomes available.[4]
Development of the therapy
The team of researchers, led by Prof. Zimmermann from University Medical Center Göttingen, Germany, coaxed stem cells to grow into heart muscle and connective tissue cells. They then mixed these cells with collagen gel to create patches which could be applied to the outside surface of the heart using a minimally invasive surgery.
After initial studies in vitro and in small animal models of heart failure confirmed the treatment had potential, the patches were first tested in monkeys. The team implanted the patches into six rhesus macaques with heart failure. Three of the monkeys received two patches, while the other three received five. These monkeys were also all treated with immunosuppressive drugs. A second group of seven monkeys remained untreated as a control.
The implanted cells remained smaller than the monkeys’ own heart muscle cells. However, the patches led to an improvement in heart function compared to the control monkeys, thickening the heart’s muscle and increasing its pumping power.[5]
First in-human trial
The success of the trial in monkeys led to the approval of a first-in-human phase 1/2 trial, called BioVAT-HF, which began in 2021 and is currently ongoing.[6][7][8]
The trial has so far recruited 19 patients. The first of these, a 46-year-old woman, had severe heart failure and was waiting for a heart transplant.[1] The researcher team implanted the muscle patches on her heart; she also received immunosuppressive drugs of the same type normally used for transplants. Three months later, the patient was lucky enough to be the recipient of a successful heart transplant.
Upon analysis of her old heart, scientists found that the implanted patches had survived and had formed blood vessels. In other words, they had integrated with the heart without any side effects.
The therapy is still in the early stages of research, and Zimmermann is very clear that it is not yet a replacement for a heart transplant. Rather, it is a supportive treatment for patients in advanced stages of heart failure, who are waiting for a transplant and are under palliative care.
Still, these are incredibly exciting results, which could prove to be a game-changer for the treatment of heart failure. The research team is continuing the clinical trial. In addition, they are testing new patch designs in monkeys in hopes of minimising the need for immunosuppressive drugs.
Stem cells: the future of medicine
This study is one more example of how stem cells are shaping research through their applications in regenerative medicine.
The stem cells used in this particular clinical trial are allogeneic (donor) induced pluripotent stem cells (iPSCs). When receiving a donor transplant, there is always a risk of rejection, hence the need for immunosuppressive drugs.
Your baby’s umbilical cord is a rich source of stem cells, among the most naïve and potent your baby will ever have. They could be used for similar therapies in the future, should your baby need them. What’s more, they could be used without risk of rejection: they are your baby’s own cells, their own perfect genetic match.
You only get one opportunity to collect and store these cells: in the 15 minutes after your baby is born. To find out more about how you can preserve this precious resource, fill in the form below to request your free guide to cord blood banking.
References
[2] NHS (2022). Heart failure. https://www.nhs.uk/conditions/heart-failure/
[4] NHS (2022). Treatment – Heart Failure. https://www.nhs.uk/conditions/heart-failure/treatment/
[8] Dzhk.de. (2025). DZHK-Studie BioVAT-HF-DZHK20. https://biovat.dzhk.de
A new clinical trial investigating the use of umbilical cord stem cells to treat chronic heart failure is currently underway.
The Phase II trial, which is the first of its kind in the U.S., is being carried out by researchers at the University of Louisville. It’s also the first time intravenous (IV) delivery will be trialled for the delivery of cell therapy for heart failure. [1]
It’s hoped that this pioneering approach could change the way that heart failure patients are treated.
What is heart failure?
Heart failure arises when the heart can’t pump blood around the body properly. This results in the body being unable to receive the oxygen it needs to function normally.
Heart failure can arise from various causes, with some of the most common being:
-
Cardiomyopathy: A condition where the heart’s muscular wall thickens, making it difficult for the heart to effectively pump blood throughout the body.
-
Heart Attack: A heart attack can cause lasting damage to the heart, impairing its ability to circulate blood efficiently.
-
High Blood Pressure: Chronic high blood pressure puts strain on the heart, gradually reducing its pumping efficiency.
-
Abnormal Heart Rhythms: An irregular, too slow, or too fast heartbeat can disrupt the heart’s normal pumping function. [2]