Stem Cell Blog
Употребата на матичните клетки од папочна врвца рапидно се зголемува. Пред 10 години крвта од папочна врвца можеше да лекува околу 40 состојби, но денес таа бројка е над 80. Со нетрпение очекуваме нови терапии за болести и нарушувања како што се дијабет, аутизам и мозочен удар, можете да бидете во тек со најновите случувања во регенеративната медицина на нашиот блог за матични клетки.
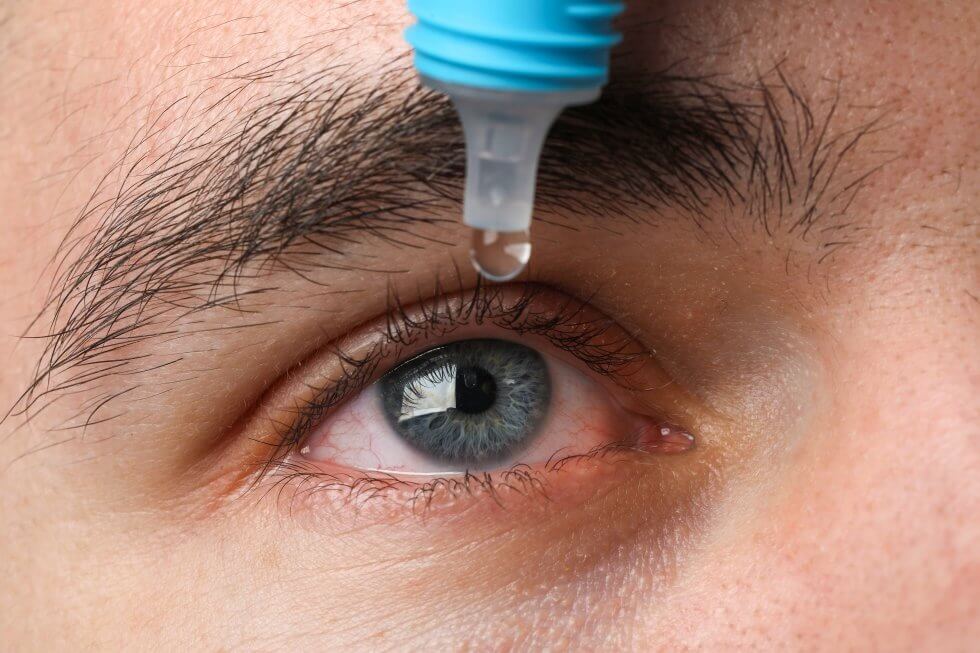
A recent study published in the Lancet journal has shown that stem cells have been used to bring about significant improvements in vision in what’s being heralded as a world first.
The stem cell treatment focused on the repair of the cornea which becomes damaged in limbal stem-cell deficiency (LSCD), a disease that can lead to blindness.
It’s hoped that these findings could have a huge impact on how those with the disease are treated and that it could underpin future therapy options for those suffering from sight loss. [1]
What is LSCD?
Limbal stem-cell deficiency (or LSCD) is a disease characterised by the loss or deficiency of stem cells in the limbus, the border between the cornea and the sclera. These stem cells are crucial to the maintenance and repair of the limbus, in addition to ensuring the continuation of its barrier function.
Problems with limbal stem cells can lead to the epithelial breakdown of the cornea resulting in inflammation, scarring and potential vision loss.
LSCD has a variety of causes ranging from genetics to acquired causes like inflammation, infection, and trauma and injury. Management of the disease differs depending on the stage of its progression.
At the early stage, managing symptoms can be sufficient to alleviate its impact on quality of life. However, more progressed instances of LSCD require surgery which usually means transplants from key parts of donor eyes. [2]
What did the stem cell study involve?
The study focused on four patients, two men and two women, aged between 39 and 72 who had all been diagnosed with LCSD in both eyes.
Researchers then derived induced pluripotent stem cells (iPSCs) from donated cord blood and used to fabricate corneal epithelial stem-progenitor cells. These were then cultured and transformed into a thin sheet, iPSC-derived corneal epithelial cell sheets (iCEPS).
After removing a layer of scar tissue covering the cornea in one eye in each of the patients, the iCEPS sheet was transplanted on top and covered with a contact lens to protect the graft.
Patients were then monitored continuously to determine safety outcomes for a period of two years. [3]
What were the results of the stem cell study?
Throughout the whole of the two year safety observation period no serious adverse events occurred. The transplant was accepted by the patients without rejection and without tumour formation. In fact, researchers reported that two of the patients even forwent immunosuppressant drugs.
Following the transplant, all four patients saw immediate improvements in their vision during the first year and three out of four patients experienced sustained improvements in their vision and quality of life beyond one year.
These results are hugely encouraging and the research team, based at Osaka University in Japan, hope to move to a larger scale clinical trial to verify their promising findings. [4]
What does this mean for cord blood banking?
As the results of this trial show, stem cells have huge regenerative potential and are at the forefront of medicine, with researchers still coming to terms with the breadth of their applications.
While this study focused on the use of induced pluripotent stem cells derived from cord blood, other types of stem cells can be found in the umbilical cord and placenta, such as haematopoietic and mesenchymal stem cells, that similarly have enormous potential in the field of regenerative medicine.
Unfortunately, the umbilical cord and placenta are often regarded as mere medical waste, meaning that these stem cells get thrown away. But by storing these stem cells in a process called cord blood banking, you can ensure that your baby always has their own stem cells available for use in future therapies.
If you or someone you know is expecting and wants to know more about the power of stem cells and how they can be stored for future use, fill out the form below to request a free Welcome Pack.
References
[1] Soma, Takeshi et al. (2024). ‘Induced pluripotent stem-cell-derived corneal epithelium for transplant surgery: a single-arm, open-label, first-in-human interventional study in Japan’. The Lancet. doi: 10.1016/S0140-6736(24)01764-1. https://doi.org/10.1016/S0140-6736(24)01764-1
[2] Karakus, S. (2024, August 2). Limbal Stem Cell Deficiency. American Academy of Opthalmology. https://eyewiki.org/Limbal_Stem_Cell_Deficiency
[3] Mallapaty, S. (2024, November 8). World-first stem-cell treatment restores vision in people. Nature. https://www.nature.com/articles/d41586-024-03656-z
[4] Mahindra, A. S. (2024, November 10). Biggest Blindness Breakthrough: Japan Performs World’s First Stem Cell-treatment To Restore Vision. Times Now. https://www.timesnownews.com/health/biggest-blindness-breakthrough-japan-performs-worlds-first-stem-cell-treatment-to-restore-vision-article-115144553
22/01/2024 BlogEye ConditionsNewsStem Cell Therapies
Medical regulators in the UK have approved a gene-editing treatment involving bone marrow stem cells designed to cure two blood diseases, including sickle cell disease, in what is a world first.
The therapy, called Casgevy, has been given the green light by the Medicines and Healthcare products Regulatory Agency (MHRA) to treat sickle cell disease and beta thalassemia, two painful blood conditions. [1]
08/01/2024 BlogEye ConditionsNewsStem Cell Therapies