Stem Cell Blog
Употребата на матичните клетки од папочна врвца рапидно се зголемува. Пред 10 години крвта од папочна врвца можеше да лекува околу 40 состојби, но денес таа бројка е над 80. Со нетрпение очекуваме нови терапии за болести и нарушувања како што се дијабет, аутизам и мозочен удар, можете да бидете во тек со најновите случувања во регенеративната медицина на нашиот блог за матични клетки.
George Norton, Acute Lymphoblastic Leukaemia
Andrew Robinson, Arthritis
Seven-time Formula One champion, Michael Schumacher, has reportedly received a pioneering stem cell treatment for heart failure this week.
The French daily Le Parisien has reported that the 50-year-old retired German racing driver is in the cardiovascular department in Georges-Pompidou hospital. Whilst there, he will receive treatment by well-known clinical cardiac surgeon, Philippe Menasché.
Professor Menasché is renowned for his long-standing interest and devotion to researching stem cell treatments for heart failure, and has been described as a ‘pioneer in cell surgery’.
It is said that Schumacher is receiving stem cell transfusions which will produce an anti-inflammatory effect throughout his entire body. But what does this mean exactly and how does it work?
Stem cell therapy
Stem cells have been referred to as ‘the building blocks of life’, and rightly so. These cells are extremely powerful and unique. They are unspecialised, which means that they can differentiate and divide into various cell types, including brain, muscle and blood cells.
Stem cell therapy, a type of regenerative medicine, promotes the repair, regrowth and regeneration of damaged or diseased tissue within the human body. Stem cells essentially target the damaged area and develop into the cells that are needed to recover.
How do stem cells help heart conditions?
The heart is arguably one of the most complex and vital organs contained within the human body and is comprised primarily of cardiac muscles cells called cardiomyocytes. These cells have very little regenerative capabilities and are often unable to heal on their own unless a heart transplant is performed.
However, thanks to incredible advancements in medicine and technology, stem cells treatments now offer a way to supply the damaged area of the heart with stem cell-derived cardiomyocytes that replace damaged tissue with healthy, beating tissue.
Schumacher’s surgeon, Professor Philippe Menasché, made medical history in 2014 when he used stem cells to do just this. Surgery was performed on a 68-year-old patient who had heart failure.
As part of the stem cell treatment, Menasché embedded millions of stem cells in a ‘patch’ attached to the heart, which enabled a new layer of healthy heart muscle to grow and leading to a significant increase in heart muscle strength and function.
The trial was an overall success, resulting in noteworthy improvements to the patient’s condition and creating a momentous victory within the medical landscape.
million people living with heart and circulatory disease in the UK: 7.4
of all deaths in the UK are caused by heart diseases and conditions: 25%
hospital admissions each day are due to heart attacks: +280
What else can stem cell therapies treat?
Stem cells can not only be used to treat heart conditions but they also have the ability to treat over 80 other diseases and conditions. They have the potential to act as a powerful tool for treating diseases, disorders and conditions
Many other stem cell therapies are currently in clinical trial stages and are being investigated in studies to treat everything from autism and cerebral palsy to hearing loss, providing exciting and potentially life-changing developments for future medical use.
Other sportspeople using stem cell treatments
Alongside Michael Schumacher, stem cell treatments are becoming increasingly popular within the world of sport, providing treatments for injuries and speedy recovery times. Tennis stars such as Rafael Nadal have used stem cell injections to facilitate recovery from back pain. Other athletes such as legendary baseball player, Bartolo Colon, received stem cell therapy to treat collateral ligament tears in his elbow, which ‘saved’ his career.
Schumacher will reportedly leave the Parisian hospital two days after his stem cell therapy. His family have released a statement saying that he is in “the very best of hands”. We wish him all the best in a speedy recovery.
References:
https://www.ncbi.nlm.nih.gov/pubmed/24835485
https://www.uab.edu/engineering/bme/people/faculty/phillipe-menasche
https://www.cirm.ca.gov/patients/power-stem-cells
http://www.isscr.org/professional-resources/news-publicationsss/isscr-news-articles/blog-detail/stem-cells-in-focus/2019/02/08/enlisting-stem-cells-in-the-war-on-heart-disease
https://www.closerlookatstemcells.org/stem-cells-medicine/heart-disease/#understand
https://www.businessinsider.com/bartolo-colons-surgery-2011-5?r=US&IR=T https://www.bioxcellerator.com/blog/5-famous-athletes-who-have-used-treatment-with-stem-cells
https://www.telegraph.co.uk/news/2019/09/10/michael-schumacher-paris-hospital-stem-cell-therapy/
https://metro.co.uk/2019/09/10/michael-schumacher-admitted-paris-hospital-stem-cell-treatment-10714998/
https://www.bhf.org.uk/for-professionals/press-centre/facts-and-figures
With Wimbledon underway once again this summer, we’ve taken a look at the ways stem cells are revolutionising one of Britain’s best-loved sports: tennis.
The origins of the sport date back thousands of years and in the 1500s, both Henry VII and Henry VIII were fans. They both commissioned the creation of tennis courts across England, and the sport has since become one of the nation’s favourites.
Yet tennis comes with a downside: it is a fast sport played on a variety of surfaces, and can result in a range of serious sports injuries. Between 2003 and 2012, an injury occurred in 20 of every 1000 sets played.
Just last year, Andy Murray looked set to retire due to a hip injury and others, such as Rafael Nadal, have similarly suffered for the sport.
What are some common tennis injuries?
The most common conditions relating to the sport are tendon damage and more specifically, ‘tennis elbow’.
The painful condition is a form of tendonitis, which causes discomfort on the outside of the elbow. It’s estimated that up to 50% of tennis players will experience this condition during their time playing the sport [2]. It also affects 1-3% of the population at large.
Could Stem Cell Injections Give Tendonitis the Elbow?
In cases of tendonitis, poor blood supply limits the amount of nutrients and growth factors that can be delivered to the site of the injury, which impairs the body’s ability to heal itself.
Damaged tendons can be treated in a variety of ways, but they are notoriously difficult to heal. For some patients, treatments are unsuccessful, and surgery may be required.
However, stem cell injections could offer an alternative option. A recent study on a group of 30 patients found that just one injection of stem cells from bone marrow to treat tennis elbow showed significant improvement in short- and medium-term follow-ups.[3]
What are the symptoms of tendonitis?
-
Tendon pain (in your knee, elbow or shoulder) which is exacerbated by movement
-
Difficulty with moving the tendon
-
A grating or crackling sensation when the tendon moves
-
Swelling, heat or redness
-
A lump along the tendon

Credit: Rafael Nadal (ESP) def. John Isner (USA), Roland Garros 2011 – mardi 24 mai – 1er tour – Court Philippe Chatrier
Stem Cells Ensure Lumbar Pain Backs Off
Lower back pain is another common complaint for tennis players. Rafael Nadal, for example, received stem cell therapy for an injury sustained to his back in 2014.
Nadal’s treatment involved cultivating his stem cells and injecting them into a joint in his spine to regenerate his cartilage. There was also hope that they would have an anti-inflammatory effect.[4]
Today, there are 39 clinical trials investigating the application of stem cells in back pain.[5]
Stem Cell Therapy Gives Rotator Cuff Injuries the Cold Shoulder
Rotator cuff injuries in tennis players are generally caused by the stress of repetitive movements to the ball and socket joint – particularly tennis strokes and the initial serve.
Treatment for an injured rotator cuff depends on the type and severity of the injury. Where muscles have been torn, surgery may be required – especially if a tear is the result of a sudden injury or if there has been no improvement after physiotherapy and steroid injections.[6]
Unfortunately, despite the improvement of surgical techniques, 25% of rotator cuff injuries will re-tear after surgery.[7] However, a study using stem cell injections during surgery showed significant healing in patients with this problem.
The study used mesenchymal stem cells that were taken from bone marrow, and found that 87% of patients treated with stem cells still had their rotator cuffs intact after ten years, compared to just 44% of the control group.[8]
Stem cells take the upper hand against knee and hip pain
In recent years, Andy Murray has famously suffered with a severe hip injury that has jeopardised his career and almost forced him into early retirement.
While the tennis ace has said traditional surgeries were insufficient for his injury, it has been reported that stem cells could be the answer. Today, regenerative medicine clinics inject stem cells into patients with joint osteoarthritis as it forms new cartilage.
One recent study treated 12 patients with moderate-to-severe knee osteoarthritis and found that they experienced a significant reduction in pain and an increase in quality of life after one year of receiving stem cells.[9]
Stem Cells Therapies Serve Additional Support
Stem cell treatments are rising through the ranks to give players more effective personalised treatments, and longevity in their careers. The potential of regenerative treatments cannot be underestimated, especially for those who play sports at a professional level.
Difficult-to-heal-injuries may no longer be career-ending, as pioneering stem cell treatments are improving healing outcomes.
Their uses extend well beyond tennis. Other athletes such as Cristiano Ronaldo, David Beckham and golf pro Jack Nicklaus have also recovered from injuries thanks to a stem cell therapy and as regenerative medicine progresses, it is likely that treatments will become available to more and more people.
References
[1] http://www.forbes.com/sites/monteburke/2012/05/30/what-is-the-most-prestigious-grand-slam-tennis-tournament/#57fd281c5666
[2] https://www.bmihealthcare.co.uk/health-matters/consultant-qa/tennis-injuries
[3] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4121921/
[4] https://www.theguardian.com/sport/2014/nov/10/rafael-nadal-stem-cell-treatment-back
[5] https://clinicaltrials.gov/ct2/results?term=back+pain+stem+cells&Search=Search
[6] http://patient.info/health/rotator-cuff-disorders
[7] http://www.medscape.com/viewarticle/842245
[8] http://www.ncbi.nlm.nih.gov/pubmed/24913770
Stem cell therapy has the potential to cure sepsis, according to a team of scientists in Durham, North Carolina.
What is sepsis and how do you get it?
Sepsis is a condition that occurs when the body’s normal response to infection goes into overdrive and causes organ dysfunction.
It is usually caused by infection. A relatively common illness such as a skin infection or pneumonia could trigger the condition.
When this happens, the body’s immune system can become overwhelmed by infection and responds with an excessive inflammatory response. This inflammation spreads and affects otherwise healthy tissue and organs.
Who is most at risk of contracting sepsis?
Sepsis is a major cause of death in premature babies, but it can affect people of all ages.
What are the signs of sepsis?
The signs of sepsis vary between newborns and adults, but generally include weakness, loss of appetite as well as fever and chills.
In babies, you can also look out for a heart rate above 90 BPM, and a breathing rate above 20 breathes per minute. It is worth keeping an eye out for these signs when an infection first begins.
The three stages of sepsis
Stage 1
-
A part of the body becomes infected
-
Germs and toxins produced by the bacteria or virus enter the bloodstream
-
This initiates an inflammatory response.
Stage 2
-
Individual organs in the body become affected.
-
They deteriorate and in some cases, fail.
Stage 3
-
In severe cases, multiple organs stop functioning
-
The patient experiences cardio-circulatory failure
-
A rapid drop in blood pressure occurs, called “septic shock”.
What is the cure for sepsis?
Currently, doctors treat sepsis with a course of antibiotics, but doctors worry that the increasing likelihood of antibiotic resistance will render this treatment inert.
The clinical study, spearheaded by Desiree Perlee from The University of Arizona Medical Center, aims to use stem cells as a potential cure for the condition.
In the trial, researchers gave 32 volunteers a drug to induce endotoxemia, a form of bacterial inflammation that has similar characteristics to sepsis and can therefore be used as a stand-in.
After an hour, doctors gave patients an infusion of allogeneic mesenchymal stem cells (ACSs), which can be found in the umbilical cord.
The results showed that these stem cells were well tolerated and at the highest dose, had promising anti-inflammatory effects as well as a mild pro-coagulant impact.
However, researchers noted that endotoxemia may respond differently to treatment and researchers need to investigate further in sepsis patients.
-
Sepsis kills 1 in every 4 patients who contract it
-
It affects 250,000 children each year in the UK
-
Kills 5 people every hour
-
44,000 deaths per year – more than bowel, breast and prostate cancer combined
-
6 million death toll worldwide
-
30 million cases of sepsis globally
Stem cells and inflammation
This trial is just the latest attempting to harness the anti-inflammatory power of stem cells to treat a life-threatening condition. Scientists are also exploring MSCs for the treatment of Alzheimer’s.
Stem cells derived from adipose tissue could play a crucial role in treating atopic dermatitis, which is more commonly known as eczema.
Scientists in Korea have successfully used exosomes in these stem cells to ameliorate symptoms of the skin condition, which affects up to 15 million people in the UK.
What is atopic dermatitis?
Atopic dermatitis, also known as eczema, is a chronic or relapsing inflammatory skin disease resulting in dry, crusty and itchy red skin. The condition is associated with elevated immunoglobin levels and sensitisation to a variety of different allergens.
It is caused by dysfunctional regulation of the skin’s immune system due to genetic and environmental factors.
How is atopic dermatitis currently treated?
Current treatments generally focus on suppressing the immune system with drugs, but these can have a negative impact in the long-term. Patients are often prescribed with topical steroid cream but these can have damaging effects.
What did the trial involve?
The trial sought to relieve atopic dermatitis by using mesenchymal stem cell-derived exosomes.
In the trial, mice were administered with varying doses of exosomes – either intravenously or subcutaneously for three times a week, over a four-week period.
What are exosomes?
Exosomes are a type of nanovesicle, a kind of liquid sac inside a cell that transports materials from one place to another. Exosomes can very easily circulate through the body to reach injury sites. They are non-toxic, even with repeated administration.
What were the results?
Researchers found that ASC exosomes reduced pathological symptoms of atopic dermatitis, such as immunoglobin levels and the number of eosinophils in the blood, without affecting overall white blood cell count. Eosinophils are disease-fighting white cells.
The stem cells also reduced the infiltration of mast cells, which release the histamine and other substances during inflammatory or allergic responses that can manifest s dermatitis.
Finally, these stem cells reduced tumour necrosis factor alpha – which are cell-signalling proteins (cytokines) that cause systemic inflammation.
How can stem cells treat eczema?
There are eight clinical trials studying the impact of stem cells on eczema.
Previous studies have indicated that MSCs derived from cord blood could suppress allergic progress in atopic dermatitis. They have the ability to interact with cell types of both innate and adaptive immune systems, which results in the differentiation and activation of immune cells – including T Cells, B Cells, dendritic cells, and natural killer cells.
However, there are issues to explore – such as improving their poor engraftment efficiency, unwanted immune response, potential tumour formation, and shorter half-life.
A promising clinical trial at Seoul St Mary’s Hospital, Korea – in association with Seoul National University – saw great results by inserting umbilical cord mesenchymal stem cells subcutaneously.
55% of patients saw a 50% reduction on the Eczema Area and Severity Index (EASI) and a 50% reduction in SCORAD (Severity Scoring for Atopic Dermatitis). Those who received higher doses also experienced a 50% reduction in itchiness.
Future treatments
Exosomes derived from stem cells could be a promising alternative therapy as they can ameliorate atopic dermatitis-type symptoms by regulating inflammatory responses. In this case, stem cells were extracted from adipose (ASCs) but can also be found in the umbilical cord.
Sleeping inside an incubator might seem like a lonely way to start life, but now premature babies can enjoy some much-needed cuddly companionship from an early age.
Volunteers have begun crocheting soft octopuses for premature babies over the past few years, with research suggesting it can soothe their early life anxiety.
With more than 60,000 premature births in the UK each year, the project offers hope to parents while they are separated from their baby.
Why octopuses?
Cuddly octopuses – and more recently, jellyfish – emulate the shape of the umbilical cord and thereby evoke feelings of warmth and safety in premature babies.
What are the benefits?
Research in Denmark has found that squeezing these cotton tentacles has helped tiny patients to develop better breathing, more regular heartbeats, and even higher levels of oxygen in their blood.
Doctors report that babies are also less likely to pull on their monitors and tubes, which ensures they can receive all the medical attention they need.
How can I make a crocheted octopus?
Crocheting guides for making your own octopus or jellyfish are freely available online.
However, it is important to note that safety precautions must be taken. The stitching pattern must be tiny to prevent stuffing from escaping and the tentacles must be just the right size – around 22cm long.
Only 100% cotton can be used, and toys must be washed regularly at a temperature above 60°C to ensure they are sterile.
How can I donate a crocheted octopus?
Several voluntary groups are active around the world, particularly in the UK, USA, Denmark, Poland and Austria.
In the UK, Octopus for Preemies has more than 23,000 volunteers. The organisation provides toys to maternity wards across the country, and has found that both parents and babies benefit from the project.
Why is the umbilical cord so special?
The positive effect of these crocheted keepsakes reaffirms the importance of the umbilical cord, which links a baby with the placenta in the womb and pumps life-giving substances from mother to child.
Find out about the power of banking your baby’s umbilical cord blood and tissue.

Since leaving hospital, premature twins, Eleanor & Isabelle continue to have a close bond with their crocheted octopuses.
Sources
https://stv.tv/news/features/1421112-cuddly-octopuses-help-scotland-s-premature-babies-feel-safe/
https://www.mirror.co.uk/lifestyle/family/how-knitted-octopus-toys-helping-9798842
Expecting a baby is exciting, let alone two or more, but there is a little more to think about when it comes to twins, not least when it comes to planning. Two car seats, two cots, a double pushchair not to mention the additional laundry and nappies! But what about cord blood banking – is it even possible? Will there be enough blood to collect?
The answer, is yes.
Cord blood collection for twins
Identical or fraternal, one placenta or two – cord blood banking fits around your birth plan and your pregnancy. Below are the top three questions asked by parents.
Do I need to bank cord blood for both twins?
This is one of the most common questions we hear, and we would always recommend you do.
Non-identical twins, are just the same as any other siblings except they happen to be born at the same time. Each is unique with their own DNA and a 1 in 4 chance of being a perfect match to the other. In contrast, identical twins will have identical DNA. However, the number of treatments available is dependent on number of stem cells, so it is important to collect as many as possible.
By storing for both twins you maximise the treatment opportunities available to your children.
Will there be enough cord blood with twins?
Twins tend to be smaller than single births as they share a womb, this, combined with smaller or a shared placenta can mean that the volume of blood available for collection can be lower.
Fortunately, Cells4Life offers a unique cord blood processing technology – CellsPlus, powered by TotiCyte. Unlike other cord blood banks, Cells4Life can process very small volumes of blood and it delivers 3x the number of cells at the point of treatment – making it equivalent to a much larger sample processed using any other system. Only Cells4Life is able to offer this service to parents expecting twins and triplets.
Will it be double the cost?
Not at all, twice the number of babies does not mean twice the price. We offer a 50% discount on the cost of cord blood processing for our Cells and Cells with Cord services for subsequent children in the same pregnancy. You can receive a personalised pricing breakdown from one of our advisors by calling 01444 873950.
Fraternal twins
Non-identical (dizygotic) twins occur when two eggs are fertilised by different sperm. Each baby will have a separate placenta, inner membrane (amnion) and outer membrane (chorion)
Identical twins
Monozygotic twins occur when one embryo splits in two. There are three different types of identical twins depending on what they share in the womb
Dichorionic diamniotic (DCDA) twins, account for almost a third of identical twins – each twin will have their own placenta, inner and outer membrane
Monochorionic diamniotic (MCDA) twins, share the same placenta and outer membrane but have their own amnion – this accounts for two thirds of identical twins.
Monochorionic monoamniotic (MCMA) twins, represent just 4% of identical twins and they share everything.
Anxiety goes hand-in-hand with parenting. From the moment you leave the hospital with your bundle of joy, to your teenager going on their first holiday with friends or moving out of home.
And that anxiety can begin long before the birth of your baby. The list of things parents-to-be need can seem never-ending; from cribs, muslins and clothes to car seats and buggies. But one thing many parents may not realise is definitely worth investing in is a paediatric first aid class. The skills they will learn will stay with them for much longer than a changing bag or bouncy chair.
Every parent will benefit from investing in this type of course. It can do many things for you, beyond just teaching you how to react in an emergency.
For new parents, every little detail is critical and worrisome. A first aid class teaches parents how to spot and deal with everything from a fever to meningitis. Having this type of training can give a parent the confidence to make better decisions for the child in that moment. Instead of panicking and worrying about what to do, the parent knows how to spring into action to provide the individual with the care he or she needs in that second. This saves lives.
New babies are rarely far from their parents so it is likely they will be the first responders in times of emergency. This is especially important in time-sensitive situations such as choking, drowning, strangulation, or a bleeding wound when it’s crucial for you to act fast and act right.
A first aid class can also teach new parents about how to baby-proof their home, which is where many accidents occur. The living room or dining room is where most accidents happen but it is the stairs or kitchen where the most serious incidents take place. A class can help you prevent and deal with scalds and burns, poisoning by bleach or medicines, falls and choking.
It will also teach you about the less obvious dangers to consider such as how dummies can be a health risk and what to consider when using car seats.
Every child is bound to get hurt at some point because they are always on the go. Life is a big adventure, with so much to explore and examine. As your child learns about its surroundings, so should you learn how to protect them.
About Daisy First Aid:
Mother of three Jenni Dunman set up Daisy First Aid four years ago after she stopped her friend’s daughter from choking on a cookie in a cafe. Working as a police officer at the time, Jenni realised that many parents lacked the first aid skills she had and wanted to do something to help educate and empower them.
Jenni has grown her business from tabletop to a multi-award winning company. She is now considered a paediatric first aid expert, featuring in numerous publications and working with celebrity parents to promote the importance of this training.
Jenni says: “We train thousands of parents every year and when you get that call to say a parent saved their child’s life thanks to our class, that is the most unbelievably emotional feeling. We are all so passionate about the importance of first aid and absolutely love what we do.”
Daisy First Aid offers fun and fear-free first aid courses designed specifically for parents, grandparents and child carers. In a two hour class, which takes place in the attendee’s own home or local venue, the world of emergency first aid unfolds. Participants interact and learn the skills they need to save a life and to treat the most common accidents and emergencies with CPR, anaphylaxis, seizures and meningitis, just some of the issues covered.
Babies and breastfeeding are very welcome too.
For over four years Cells4Life has offered the additional option of storing umbilical cord tissue stem cells alongside those found in the cord blood. But what is so special about these cells and why is it important to preserve them?
The benefits of storing umbilical cord tissue stem cells
More cell types
Cord tissue is one of the richest sources of mesenchymal stem cells available. These are the cells that are thought to have the most potential in the field of regenerative medicine.
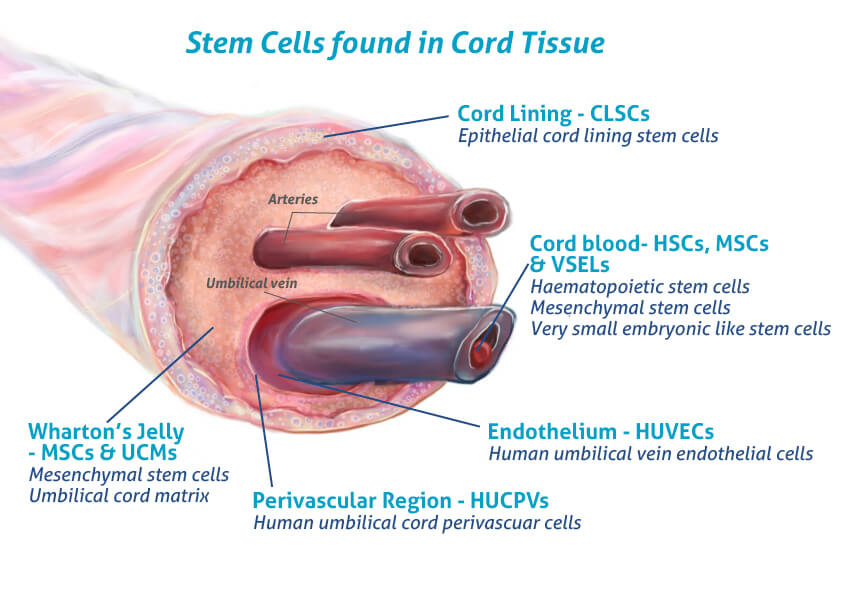
Mesenchymal Stem Cells (MSCs)
The MSCs found in the ‘Wharton’s Jelly’ are more abundant and powerful than those in the cord blood and have huge potential for use in a wide range of regenerative therapies.
http://www.ncbi.nlm.nih.gov/pubmed/20187873
Human Umbilical Cord Perivascular cells (HUCPVCs)
The perivascular cells found in the umbilical cord are being investigated for bone formation and dermal tissue engineering.
http://www.ncbi.nlm.nih.gov/pubmed/25676366
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3055644
Human Umbilical Vein Endothelial Cells (HUVECs)
These cells are easy to culture and commonly used in a research setting for vascular biology – including inflammation, blood clotting and angiogenesis.
http://www.ncbi.nlm.nih.gov/pubmed/17237547
Epithelial Cord Lining Stem Cells (CLSCs)
CLSCs have proved effective in the treatment of difficult to heal human wounds such as diabetic ulcers, defects to the cornea and have demonstrated successful regeneration of the liver and heart in animal studies.
http://www.ncbi.nlm.nih.gov/pubmed/24636
Keep it in the family
Not only will the umbilical cord tissue stem cells be a 100% match to your child – they could also be match to other members of the family too.
Readily available
One of the great advantages of umbilical cord tissue stem cells is the ease by which they can be collected. In the very unlikely event that there is any difficulty with the collection of cord blood, stem cells from the umbilical cord tissue will almost certainly still be available.
“Our umbilical cord tissue samples are processed and stored exclusively by Cells4Life here in the UK”
Uses of umbilical cord tissue stem cells
Cord tissue is currently being investigated for use in the treatment of the following conditions:
• Alzheimer’s disease
• Aplastic anaemia
• Cardiomyopathy
• Cartilage repair
• Cerebral Palsy
• Connective tissue diseases
• Diabetes (type 2)
• Erectile Dysfunction
• Liver failure
• Multiple Sclerosis
• Myocardial infarction
• Osteoarthritis
• Ovarian failure
• Parkinson’s disease
• Psoriasis
• Retinitis pigmentosa
• Rheumatoid arthritis
• Sepsis
• Spinal cord injury
• Stroke
• Traumatic optic neuropathy
• Ulcerative colitis
• And many more…
“In 2014 Cells4Life became the first private cord blood bank to provide cord tissue for an experimental treatment of a skin condition at Guys and St Thomas’ NHS Hospital in London.”
Down’s syndrome is a genetic disorder caused by an abnormality, involving chromosome 21. The condition results in impaired cognitive development and a distinctive physical appearance. Scientists don’t yet truly understand the underlying cause of Down’s syndrome and in 94% of cases it likely simply to have been a fluke of nature.

In the UK, 2 babies are born with Down’s syndrome every day.

There are currently 60,000 people in Britain with the condition.
There are 3 types of test for Down’s syndrome.
How can I test for Down’s syndrome?
Currently there are three main types of test available and they can be broken down into two categories: screening and diagnostic testing. The Combined Test and NIPT are screenings and indicate the risk of a baby having Down’s syndrome, whereas Amniocentesis and CVS provide a definitive diagnosis.
Combined Test
This test is available to all expectant mothers from 10-14 weeks of pregnancy and gives a result based on a combination of the mother’s age, an ultrasound scan, and a blood test. The Combined Test has a detection rate of 90%, however, 1 in 20 positive results will be false. This means 5% of parents are left worrying without reason and may evening undergo the far riskier Amniocentesis or CVS tests unnecessarily as a result.
Non-Invasive Prenatal Testing (NIPT)
This newer kind of test is not yet available on the NHS, however, it can be requested through private clinics. It involves a simple blood test that analyses cell free DNA found in the mother’s plasma. NIPT offers a detection rate of >98% and up to a 0.01% false positive rate, making it far more accurate than the Combined Test.
Chorion Villus Sampling (CVS)/ Amniocentesis
These invasive tests follow a positive Combined Test or non–invasive screening and involve passing a fine needle through the mother’s abdomen to collected a sample of cells from either the placenta or surrounding fluid. Whilst both tests deliver an accurate diagnosis of the condition they are not without risk – the procedure carries a 1% chance of causing miscarriage.
Non-invasive prenatal testing with Cells4Life
There are a variety of NIPT services available that test for Down’s syndrome and much more. These include Panaroma, Harmony, NIFTY and Genesis Serenity
Cells4Life offer the Panoroma test as it is the most accurate Down’s Syndrome screening available, with a detection rate of >99%. It is also screens for several conditions that other tests do not, including triploidy, molar pregnancy and vanishing twin, and is the only test to differentiate between maternal and fetal DNA, resulting in a significant reduction is false positives.
The Panorama screens for the following conditions:

Chromosome Abnormalities:
-
-
Trisomy 21 (Down syndrome)
-
Trisomy 18 (Edwards syndrome)
-
Trisomy 13 (Patau syndrome)
-
Triploidy
-

Sex Chromosome Abnormalities:
-
Monosomy X (Turner syndrome)
-
Klinefelter syndrome
-
Triple X syndrome
-
XYY syndrome

Microdeletions:
-
-
-
22q11.2 deletion syndrome
-
1p36 deletion syndrome
-
Prader-Willi syndrome
-
Angolan syndrome
-
Cri-du-chat syndrome
-
Gender of the Baby (optional)
-
-
Learn more about non-invasive prenatal testing and our test for down’s syndrome on on our information pages.
References